Abstract
Epidermolysis bullosa (EB) is a heterogeneous group of rare inherited blistering skin disorders characterized by skin fragility following minor trauma, usually present since birth. EB can be categorized into four classical subtypes, EB simplex, junctional EB, dystrophic EB and Kindler EB, distinguished on clinical features, plane of blister formation in the skin, and molecular pathology. Treatment for EB is mostly supportive, focusing on wound care and patient symptoms such as itch or pain. However, therapeutic advances have also been made in targeting the primary genetic abnormalities as well as the secondary inflammatory footprint of EB. Pre-clinical or clinical testing of gene therapies (gene replacement, gene editing, RNA-based therapy, natural gene therapy), cell-based therapies (fibroblasts, bone marrow transplantation, mesenchymal stromal cells, induced pluripotential stem cells), recombinant protein therapies, and small molecule and drug repurposing approaches, have generated new hope for better patient care. In this article, we review advances in translational research that are impacting on the quality of life for people living with different forms of EB and which offer hope for improved clinical management.


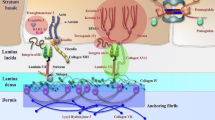
modified from Bardhan et al. [2]. BP180 bullous pemphigoid antigen 180, BP230 bullous pemphigoid antigen 230, CD151 cluster of differentiation 151, DEB dystrophic EB, EB epidermolysis bullosa, EBS EB simplex, JEB junctional EB, KEB Kindler EB, KLHL24 kelch-like family member 24



Similar content being viewed by others
References
Baardman R, Yenamandra VK, Duipmans JC, Pasmooij AMG, Jonkman MF, van den Akker PC, et al. Novel insights into the epidemiology of epidermolysis bullosa (EB) from the Dutch EB Registry: EB more common than previously assumed? J Eur Acad Dermatol Venereol. 2021;35:995–1006.
Bardhan A, Bruckner-Tuderman L, Chapple ILC, Fine JD, Harper N, Has C, et al. Epidermolysis bullosa. Nat Rev Dis Prim. 2020;6:78.
Has C, Bauer JW, Bodemer C, Bolling MC, Bruckner-Tuderman L, Diem A, et al. Consensus reclassification of inherited epidermolysis bullosa and other disorders with skin fragility. Br J Dermatol. 2020;183:614–27.
Mavilio F, Pellegrini G, Ferrari S, Di Nunzio F, Di Iorio E, Recchia A, et al. Correction of junctional epidermolysis bullosa by transplantation of genetically modified epidermal stem cells. Nat Med. 2006;12:1397–402.
Hirsch T, Rothoeft T, Teig N, Bauer JW, Pellegrini G, De Rosa L, et al. Regeneration of the entire human epidermis using transgenic stem cells. Nature. 2017;551:327–32.
De Rosa L, Carulli S, Cocchiarella F, Quaglino D, Enzo E, Franchini E, et al. Long-term stability and safety of transgenic cultured epidermal stem cells in gene therapy of junctional epidermolysis bullosa. Stem Cell Rep. 2014;2:1–8.
Barrandon Y, Green H. Three clonal types of keratinocyte with different capacities for multiplication. Proc Natl Acad Sci USA. 1987;84:2302–6.
Siprashvili Z, Nguyen NT, Gorell ES, Loutit K, Khuu P, Furukawa LK, et al. Safety and wound outcomes following genetically corrected autologous epidermal grafts in patients with recessive dystrophic epidermolysis bullosa. JAMA. 2016;316:1808–17.
Eichstadt S, Barriga M, Ponakala A, Teng C, Nguyen NT, Siprashvili Z, et al. Phase 1/2a clinical trial of gene-corrected autologous cell therapy for recessive dystrophic epidermolysis bullosa. JCI insight. 2019;4(19):e130554.
Gaucher S, Lwin SM, Titeux M, Abdul-Wahab A, Pironon N, Izmiryan A, et al. EBGene trial: patient preselection outcomes for the European GENEGRAFT ex vivo phase I/II gene therapy trial for recessive dystrophic epidermolysis bullosa. Br J Dermatol. 2020;182:794–7.
Marinkovich M, Lane A, Sridhar K, Keene D, Malyala A, Maslowski J. 591 A phase 1/2 study of genetically-corrected, collagen VII expressing autologous human dermal fibroblasts injected into the skin of patients with recessive dystrophic epidermolysis bullosa (RDEB). J Invest Dermatol. 2018;138:S100.
Lwin SM, Syed F, Di WL, Kadiyirire T, Liu L, Guy A, et al. Safety and early efficacy outcomes for lentiviral fibroblast gene therapy in recessive dystrophic epidermolysis bullosa. JCI Insight. 2019;4:e126243.
Marinkovich MP, Vinzant S, Karkala V, Sridhar K, Gurevitch I, Dolorito J, et al. 305 In vivo correction of recessive dystrophic epidermolysis bullosa (RDEB) by direct cutaneous COL7A1 gene replacement: results of a phase 1–2 trial. J Investig Dermatol. 2020;140:S37.
Marinkovich MP, Tang JY. Gene therapy for epidermolysis bullosa. J Investig Dermatol. 2019;139:1221–6.
Osborn MJ, Starker CG, McElroy AN, Webber BR, Riddle MJ, Xia L, et al. TALEN-based gene correction for epidermolysis bullosa. Mol Ther. 2013;21:1151–9.
Melo SP, Lisowski L, Bashkirova E, Zhen HH, Chu K, Keene DR, et al. Somatic correction of junctional epidermolysis bullosa by a highly recombinogenic AAV variant. Mol Ther. 2014;22:725–33.
Sebastiano V, Zhen HH, Haddad B, Bashkirova E, Melo SP, Wang P, et al. Human COL7A1-corrected induced pluripotent stem cells for the treatment of recessive dystrophic epidermolysis bullosa. Sci Transl Med. 2014;6:264ra163.
Chamorro C, Mencia A, Almarza D, Duarte B, Buning H, Sallach J, et al. Gene editing for the efficient correction of a recurrent COL7A1 mutation in recessive dystrophic epidermolysis bullosa keratinocytes. Mol Ther Nucleic Acids. 2016;5:e307.
Izmiryan A, Danos O, Hovnanian A. Meganuclease-mediated COL7A1 gene correction for recessive dystrophic epidermolysis bullosa. J Investig Dermatol. 2016;136:872–5.
Shinkuma S, Guo Z, Christiano AM. Site-specific genome editing for correction of induced pluripotent stem cells derived from dominant dystrophic epidermolysis bullosa. Proc Natl Acad Sci USA. 2016;113:5676–81.
Aushev M, Koller U, Mussolino C, Cathomen T, Reichelt J. Traceless targeting and isolation of gene-edited immortalized keratinocytes from epidermolysis bullosa simplex patients. Mol Ther Methods Clin Dev. 2017;6:112–23.
Hainzl S, Peking P, Kocher T, Murauer EM, Larcher F, Del Rio M, et al. COL7A1 editing via CRISPR/Cas9 in recessive dystrophic epidermolysis bullosa. Mol Ther. 2017;25:2573–84.
Benati D, Miselli F, Cocchiarella F, Patrizi C, Carretero M, Baldassarri S, et al. CRISPR/Cas9-mediated in situ correction of LAMB3 gene in keratinocytes derived from a junctional epidermolysis bullosa patient. Mol Ther. 2018;26:2592–603.
March OP, Kocher T, Koller U. Context-dependent strategies for enhanced genome editing of genodermatoses. Cells. 2020;9:112.
Rees HA, Liu DR. Base editing: precision chemistry on the genome and transcriptome of living cells. Nat Rev Genet. 2018;19:770–88.
Komor AC, Kim YB, Packer MS, Zuris JA, Liu DR. Programmable editing of a target base in genomic DNA without double-stranded DNA cleavage. Nature. 2016;533:420–4.
Osborn MJ, Newby GA, McElroy AN, Knipping F, Nielsen SC, Riddle MJ, et al. Base editor correction of COL7A1 in recessive dystrophic epidermolysis bullosa patient-derived fibroblasts and iPSCs. J Invest Dermatol. 2020;140:338–47.
Anzalone AV, Randolph PB, Davis JR, Sousa AA, Koblan LW, Levy JM, et al. Search-and-replace genome editing without double-strand breaks or donor DNA. Nature. 2019;576:149–57.
Bornert O, Peking P, Bremer J, Koller U, van den Akker PC, Aartsma-Rus A, et al. RNA-based therapies for genodermatoses. Exp Dermatol. 2017;26:3–10.
Dallinger G, Puttaraju M, Mitchell LG, Yancey KB, Yee C, Klausegger A, et al. Development of spliceosome-mediated RNA trans-splicing (SMaRT) for the correction of inherited skin diseases. Exp Dermatol. 2003;12:37–46.
Wally V, Klausegger A, Koller U, Lochmuller H, Krause S, Wiche G, et al. 5’ trans-splicing repair of the PLEC1 gene. J Investig Dermatol. 2008;128:568–74.
Wally V, Brunner M, Lettner T, Wagner M, Koller U, Trost A, et al. K14 mRNA reprogramming for dominant epidermolysis bullosa simplex. Hum Mol Genet. 2010;19:4715–25.
Koller U, Wally V, Mitchell LG, Klausegger A, Murauer EM, Mayr E, et al. A novel screening system improves genetic correction by internal exon replacement. Nucleic Acids Res. 2011;39:e108.
Murauer EM, Gache Y, Gratz IK, Klausegger A, Muss W, Gruber C, et al. Functional correction of type VII collagen expression in dystrophic epidermolysis bullosa. J Investig Dermatol. 2011;131:74–83.
Peking P, Breitenbach JS, Ablinger M, Muss WH, Poetschke FJ, Kocher T, et al. An ex vivo RNA trans-splicing strategy to correct human generalized severe epidermolysis bullosa simplex. Br J Dermatol. 2019;180:141–8.
Coutinho MF, Matos L, Santos JI, Alves S. RNA therapeutics: how far have we gone? Adv Exp Med Biol. 2019;1157:133–77.
Bornert O, Kuhl T, Bremer J, van den Akker PC, Pasmooij AM, Nystrom A. Analysis of the functional consequences of targeted exon deletion in COL7A1 reveals prospects for dystrophic epidermolysis bullosa therapy. Mol Ther. 2016;24:1302–11.
Rodrigues M, Yokota T. An overview of recent advances and clinical applications of exon skipping and splice modulation for muscular dystrophy and various genetic diseases. Methods Mol Biol. 2018;1828:31–55.
Goto M, Sawamura D, Nishie W, Sakai K, McMillan JR, Akiyama M, et al. Targeted skipping of a single exon harboring a premature termination codon mutation: implications and potential for gene correction therapy for selective dystrophic epidermolysis bullosa patients. J Investig Dermatol. 2006;126:2614–20.
Bremer J, Bornert O, Nystrom A, Gostynski A, Jonkman MF, Aartsma-Rus A, et al. Antisense oligonucleotide-mediated exon skipping as a systemic therapeutic approach for recessive dystrophic epidermolysis bullosa. Mol Ther Nucleic Acids. 2016;5:e379.
Turczynski S, Titeux M, Tonasso L, Decha A, Ishida-Yamamoto A, Hovnanian A. Targeted exon skipping restores type VII collagen expression and anchoring fibril formation in an in vivo RDEB model. J Investig Dermatol. 2016;136:2387–95.
Bornert O, Hogervorst M, Nauroy P, Bischof J, Swildens J, Athanasiou I, et al. QR-313, an antisense oligonucleotide, shows therapeutic efficacy for treatment of dominant and recessive dystrophic epidermolysis bullosa: a preclinical study. J Investig Dermatol. 2021;141:883–93.
Marinkovich MP, Sridhar K, Karkala V, Yenamandra VK, Gurevitch I, Dolorito J, et al. 306 Topical QR-313, an antisense oligonucleotide, in the treatment of dystrophic epidermolysis bullosa. J Investig Dermatol. 2020;140:S37.
Ablinger M, Lettner T, Friedl N, Potocki H, Palmetzhofer T, Koller U, et al. Personalized development of antisense oligonucleotides for exon skipping restores type XVII collagen expression in junctional epidermolysis bullosa. Int J Mol Sci. 2021;22:3326.
Atkinson SD, McGilligan VE, Liao H, Szeverenyi I, Smith FJ, Moore CB, et al. Development of allele-specific therapeutic siRNA for keratin 5 mutations in epidermolysis bullosa simplex. J Investig Dermatol. 2011;131:2079–86.
Pendaries V, Gasc G, Titeux M, Tonasso L, Mejia JE, Hovnanian A. siRNA-mediated allele-specific inhibition of mutant type VII collagen in dominant dystrophic epidermolysis bullosa. J Investig Dermatol. 2012;132:1741–3.
Leachman SA, Hickerson RP, Schwartz ME, Bullough EE, Hutcherson SL, Boucher KM, et al. First-in-human mutation-targeted siRNA phase Ib trial of an inherited skin disorder. Mol Ther. 2010;18:442–6.
Lim YH, Fisher JM, Choate KA. Revertant mosaicism in genodermatoses. Cell Mol Life Sci. 2017;74:2229–38.
Twaroski K, Eide C, Riddle MJ, Xia L, Lees CJ, Chen W, et al. Revertant mosaic fibroblasts in recessive dystrophic epidermolysis bullosa. Br J Dermatol. 2019;181:1247–53.
Jonkman MF, Scheffer H, Stulp R, Pas HH, Nijenhuis M, Heeres K, et al. Revertant mosaicism in epidermolysis bullosa caused by mitotic gene conversion. Cell. 1997;88:543–51.
Schuilenga-Hut PH, Scheffer H, Pas HH, Nijenhuis M, Buys CH, Jonkman MF. Partial revertant mosaicism of keratin 14 in a patient with recessive epidermolysis bullosa simplex. J Investig Dermatol. 2002;118:626–30.
Smith FJ, Morley SM, McLean WH. Novel mechanism of revertant mosaicism in Dowling-Meara epidermolysis bullosa simplex. J Investig Dermatol. 2004;122:73–7.
Kiritsi D, He Y, Pasmooij AM, Onder M, Happle R, Jonkman MF, et al. Revertant mosaicism in a human skin fragility disorder results from slipped mispairing and mitotic recombination. J Clin Investig. 2012;122:1742–6.
Lai-Cheong JE, Moss C, Parsons M, Almaani N, McGrath JA. Revertant mosaicism in Kindler syndrome. J Investig Dermatol. 2012;132:730–2.
Pasmooij AM, Nijenhuis M, Brander R, Jonkman MF. Natural gene therapy may occur in all patients with generalized non-Herlitz junctional epidermolysis bullosa with COL17A1 mutations. J Investig Dermatol. 2012;132:1374–83.
van den Akker PC, Nijenhuis M, Meijer G, Hofstra RM, Jonkman MF, Pasmooij AM. Natural gene therapy in dystrophic epidermolysis bullosa. Arch Dermatol. 2012;148:213–6.
Lai-Cheong JE, McGrath JA, Uitto J. Revertant mosaicism in skin: natural gene therapy. Trends Mol Med. 2011;17:140–8.
Kiritsi D, Garcia M, Brander R, Has C, Meijer R, Jose Escamez M, et al. Mechanisms of natural gene therapy in dystrophic epidermolysis bullosa. J Investig Dermatol. 2014;134:2097–104.
Gostynski A, Deviaene FC, Pasmooij AM, Pas HH, Jonkman MF. Adhesive stripping to remove epidermis in junctional epidermolysis bullosa for revertant cell therapy. Br J Dermatol. 2009;161:444–7.
Gostynski A, Pasmooij AM, Jonkman MF. Successful therapeutic transplantation of revertant skin in epidermolysis bullosa. J Am Acad Dermatol. 2014;70:98–101.
Matsumura W, Fujita Y, Shinkuma S, Suzuki S, Yokoshiki S, Goto H, et al. Cultured epidermal autografts from clinically revertant skin as a potential wound treatment for recessive dystrophic epidermolysis bullosa. J Investig Dermatol. 2019;139:2115–24.
Tolar J, McGrath JA, Xia L, Riddle MJ, Lees CJ, Eide C, et al. Patient-specific naturally gene-reverted induced pluripotent stem cells in recessive dystrophic epidermolysis bullosa. J Investig Dermatol. 2014;134:1246–54.
Umegaki-Arao N, Pasmooij AM, Itoh M, Cerise JE, Guo Z, Levy B, et al. Induced pluripotent stem cells from human revertant keratinocytes for the treatment of epidermolysis bullosa. Sci Transl Med. 2014;6:264ra164.
Jo H, Brito S, Kwak BM, Park S, Lee MG, Bin BH. Applications of mesenchymal stem cells in skin regeneration and rejuvenation. Int J Mol Sci. 2021;22:2410.
Buzhor E, Leshansky L, Blumenthal J, Barash H, Warshawsky D, Mazor Y, et al. Cell-based therapy approaches: the hope for incurable diseases. Regen Med. 2014;9:649–72.
Rheinwatd JG, Green H. Seria cultivation of strains of human epidemal keratinocytes: the formation keratinizin colonies from single cell is. Cell. 1975;6:331–43.
Carter DM, Lin AN, Varghese MC, Caldwell D, Pratt LA, Eisinger M. Treatment of junctional epidermolysis bullosa with epidermal autografts. J Am Acad Dermatol. 1987;17:246–50.
Eisenberg M, Llewellyn DM, Moran K, Kerr A. Successful engraftment of cultured human epidermal allograft in a child with recessive dystrophic epidermolysis bullosa. Med J Aust. 1987;147:520–1.
McGrath JA, Schofield OMV, Ishida-Yamamoto A, O’Grady A, Mayou BJ, Navsaria H, et al. Cultured keratinocyte allografts and wound healing in severe recessive dystrophic epidermolysis bullosa. J Am Acad Dermatol. 1993;29:407–19.
Petrof G, Martinez-Queipo M, Mellerio JE, Kemp P, McGrath JA. Fibroblast cell therapy enhances initial healing in recessive dystrophic epidermolysis bullosa wounds: results of a randomized, vehicle-controlled trial. Br J Dermatol. 2013;169:1025–33.
Venugopal SS, Yan W, Frew JW, Cohn HI, Rhodes LM, Tran K, et al. A phase II randomized vehicle-controlled trial of intradermal allogeneic fibroblasts for recessive dystrophic epidermolysis bullosa. J Am Acad Dermatol. 2013;69:898–908.
Wong T, Gammon L, Liu L, Mellerio JE, Dopping-Hepenstal PJ, Pacy J, et al. Potential of fibroblast cell therapy for recessive dystrophic epidermolysis bullosa. J Investig Dermatol. 2008;128:2179–89.
Nagy N, Almaani N, Tanaka A, Lai-Cheong JE, Techanukul T, Mellerio JE, et al. HB-EGF induces COL7A1 expression in keratinocytes and fibroblasts: possible mechanism underlying allogeneic fibroblast therapy in recessive dystrophic epidermolysis Bullosa. J Investig Dermatol. 2011;131:1771–4.
Hsu CK, Wang SP, Lee JY, McGrath JA. Treatment of hereditary epidermolysis bullosa: updates and future prospects. Am J Clin Dermatol. 2014;15:1–6.
Leal-Marin S, Kern T, Hofmann N, Pogozhykh O, Framme C, Borgel M, et al. Human amniotic membrane: a review on tissue engineering, application, and storage. J Biomed Mater Res B Appl Biomater. 2020. https://doi.org/10.1002/jbm.b.34782.
Walkden A. Amniotic membrane transplantation in ophthalmology: an updated perspective. Clin Ophthalmol. 2020;14:2057–72.
Lo V, Lara-Corrales I, Stuparich A, Pope E. Amniotic membrane grafting in patients with epidermolysis bullosa with chronic wounds. J Am Acad Dermatol. 2010;62:1038–44.
Koulisis N, Moysidis SN, Siegel LM, Song JC. Long-term follow-up of amniotic membrane graft for the treatment of symblepharon in a patient with recessive dystrophic epidermolysis bullosa. Cornea. 2016;35:1242–4.
Moravvej H, Abdollahimajd F, Naseh MH, Piravar Z, Abolhasani E, Mozafari N, et al. Cultured allogeneic fibroblast injection vs fibroblasts cultured on amniotic membrane scaffold for dystrophic epidermolysis bullosa treatment. Br J Dermatol. 2018;179:72–9.
Falabella AF, Schachner LA, Valencia IC, Eaglstein WH. The use of tissue-engineered skin (Apligraf) to treat a newborn with epidermolysis bullosa. Arch Dermatol. 1999;135:1219–22.
Falabella AF, Valencia IC, Eaglstein WH, Schachner LA. Tissue-engineered skin (Apligraf) in the healing of patients with epidermolysis bullosa wounds. Arch Dermatol. 2000;136:1225–30.
Fivenson DP, Scherschun L, Choucair M, Kukuruga D, Young J, Shwayder T. Graftskin therapy in epidermolysis bullosa. J Am Acad Dermatol. 2003;48:886–92.
Fivenson DP, Scherschun L, Cohen LV. Apligraf in the treatment of severe mitten deformity associated with recessive dystrophic epidermolysis bullosa. Plast Reconstr Surg. 2003;112:584–8.
Chino T, Tamai K, Yamazaki T, Otsuru S, Kikuchi Y, Nimura K, et al. Bone marrow cell transfer into fetal circulation can ameliorate genetic skin diseases by providing fibroblasts to the skin and inducing immune tolerance. Am J Pathol. 2008;173:803–14.
Tolar J, Ishida-Yamamoto A, Riddle M, McElmurry RT, Osborn M, Xia L, et al. Amelioration of epidermolysis bullosa by transfer of wild-type bone marrow cells. Blood. 2009;113:1167–74.
Wagner JE, Ishida-Yamamoto A, McGrath JA, Hordinsky M, Keene DR, Woodley DT, et al. Bone marrow transplantation for recessive dystrophic epidermolysis bullosa. N Engl J Med. 2010;363:629–39.
Ebens CL, McGrath JA, Tamai K, Hovnanian A, Wagner JE, Riddle MJ, et al. Bone marrow transplant with post-transplant cyclophosphamide for recessive dystrophic epidermolysis bullosa expands the related donor pool and permits tolerance of nonhaematopoietic cellular grafts. Br J Dermatol. 2019;181:1238–46.
Gostynska KB, Yenamandra VK, Lindemans C, Duipmans J, Gostynski A, Jonkman MF, et al. Allogeneic haematopoietic cell transplantation for epidermolysis bullosa: the dutch experience. Acta Derm Venereol. 2019;99:347–8.
Vanden Oever M, Twaroski K, Osborn MJ, Wagner JE, Tolar J. Inside out: regenerative medicine for recessive dystrophic epidermolysis bullosa. Pediatr Res. 2018;83:318–24.
Geyer MB, Radhakrishnan K, Giller R, Umegaki N, Harel S, Kiuru M, et al. Reduced toxicity conditioning and allogeneic hematopoietic progenitor cell transplantation for recessive dystrophic epidermolysis bullosa. J Pediatr. 2015;167:765–9.
Ebens CL, McGrath JA, Riedl JA, Keith AR, Lilja G, Rusch S, et al. Immune tolerance of allogeneic haematopoietic cell transplantation supports donor epidermal grafting of recessive dystrophic epidermolysis bullosa chronic wounds. Br J Dermatol. 2020. https://doi.org/10.1111/bjd.19503.
Conget P, Rodriguez F, Kramer S, Allers C, Simon V, Palisson F, et al. Replenishment of type VII collagen and re-epithelialization of chronically ulcerated skin after intradermal administration of allogeneic mesenchymal stromal cells in two patients with recessive dystrophic epidermolysis bullosa. Cytotherapy. 2010;12:429–31.
Petrof G, Lwin SM, Martinez-Queipo M, Abdul-Wahab A, Tso S, Mellerio JE, et al. Potential of systemic allogeneic mesenchymal stromal cell therapy for children with recessive dystrophic epidermolysis bullosa. J Investig Dermatol. 2015;135:2319–21.
El-Darouti M, Fawzy M, Amin I, Abdel Hay R, Hegazy R, Gabr H, et al. Treatment of dystrophic epidermolysis bullosa with bone marrow non-hematopoeitic stem cells: a randomized controlled trial. Dermatol Ther. 2016;29:96–100.
Rashidghamat E, Kadiyirire T, Ayis S, Petrof G, Liu L, Pullabhatla V, et al. Phase I/II open-label trial of intravenous allogeneic mesenchymal stromal cell therapy in adults with recessive dystrophic epidermolysis bullosa. J Am Acad Dermatol. 2020;83:447–54.
Kuroda Y, Kitada M, Wakao S, Nishikawa K, Tanimura Y, Makinoshima H, et al. Unique multipotent cells in adult human mesenchymal cell populations. Proc Natl Acad Sci USA. 2010;107:8639–43.
Vander Beken S, de Vries JC, Meier-Schiesser B, Meyer P, Jiang D, Sindrilaru A, et al. Newly defined ATP-binding cassette subfamily B member 5 positive dermal mesenchymal stem cells promote healing of chronic iron-overload wounds via secretion of interleukin-1 receptor antagonist. Stem Cells. 2019;37:1057–74.
Fujita Y, Nohara T, Takashima S, Natsuga K, Adachi M, Yoshida K, et al. Intravenous allogeneic multilineage-differentiating stress-enduring cells in adults with dystrophic epidermolysis bullosa: a phase 1/2 open-label study. J Eur Acad Dermatol Venereol. 2021. https://doi.org/10.1111/jdv.17201.
Maseda R, Martinez-Santamaria L, Sacedon R, Butta N, de Arriba MDC, Garcia-Barcenilla S, et al. Beneficial effect of systemic allogeneic adipose derived mesenchymal cells on the clinical, inflammatory and immunologic status of a patient with recessive dystrophic epidermolysis bullosa: a case report. Front Med (Lausanne). 2020;7:576558.
Lee SE, Lee SJ, Kim SE, Kim K, Cho B, Roh K, et al. Intravenous allogeneic umbilical cord blood-derived mesenchymal stem cell therapy in recessive dystrophic epidermolysis bullosa patients. JCI Insight. 2021;6:e143606.
O’Brien K, Breyne K, Ughetto S, Laurent LC, Breakefield XO. RNA delivery by extracellular vesicles in mammalian cells and its applications. Nat Rev Mol Cell Biol. 2020;21:585–606.
McBride JD, Rodriguez-Menocal L, Candanedo A, Guzman W, Garcia-Contreras M, Badiavas EV. Dual mechanism of type VII collagen transfer by bone marrow mesenchymal stem cell extracellular vesicles to recessive dystrophic epidermolysis bullosa fibroblasts. Biochimie. 2018;155:50–8.
Woodley DT, Keene DR, Atha T, Huang Y, Lipman K, Li W, et al. Injection of recombinant human type VII collagen restores collagen function in dystrophic epidermolysis bullosa. Nat Med. 2004;10:693–5.
Remington J, Wang X, Hou Y, Zhou H, Burnett J, Muirhead T, et al. Injection of recombinant human type VII collagen corrects the disease phenotype in a murine model of dystrophic epidermolysis bullosa. Mol Ther. 2009;17:26–33.
Woodley DT, Wang X, Amir M, Hwang B, Remington J, Hou Y, et al. Intravenously injected recombinant human type VII collagen homes to skin wounds and restores skin integrity of dystrophic epidermolysis bullosa. J Investig Dermatol. 2013;133:1910–3.
Hou Y, Guey LT, Wu T, Gao R, Cogan J, Wang X, et al. Intravenously administered recombinant human type VII collagen derived from chinese hamster ovary cells reverses the disease phenotype in recessive dystrophic epidermolysis bullosa mice. J Investig Dermatol. 2015;135:3060–7.
South AP, Uitto J. Type VII collagen replacement therapy in recessive dystrophic epidermolysis bullosa-how much, how often? J Investig Dermatol. 2016;136:1079–81.
Bidou L, Allamand V, Rousset JP, Namy O. Sense from nonsense: therapies for premature stop codon diseases. Trends Mol Med. 2012;18:679–88.
Howard M, Frizzell RA, Bedwell DM. Aminoglycoside antibiotics restore CFTR function by overcoming premature stop mutations. Nat Med. 1996;2:467–9.
Barton-Davis ER, Cordier L, Shoturma DI, Leland SE, Sweeney HL. Aminoglycoside antibiotics restore dystrophin function to skeletal muscles of mdx mice. J Clin Investig. 1999;104:375–81.
Cogan J, Weinstein J, Wang X, Hou Y, Martin S, South AP, et al. Aminoglycosides restore full-length type VII collagen by overcoming premature termination codons: therapeutic implications for dystrophic epidermolysis bullosa. Mol Ther. 2014;22:1741–52.
Woodley DT, Cogan J, Hou Y, Lyu C, Marinkovich MP, Keene D, et al. Gentamicin induces functional type VII collagen in recessive dystrophic epidermolysis bullosa patients. J Clin Investig. 2017;127:3028–38.
Kwong A, Cogan J, Hou Y, Antaya R, Hao M, Kim G, et al. Gentamicin induces laminin 332 and improves wound healing in junctional epidermolysis bullosa patients with nonsense mutations. Mol Ther. 2020;28:1327–38.
Li Y, Shen J, Liang J, Zheng L, Chen F, Yao Z, et al. Gentamicin induces COL17A1 nonsense mutation readthrough in junctional epidermolysis bullosa. J Dermatol. 2020;47:e82–3.
Hammersen J, Neuner A, Wild F, Schneider H. Attenuation of severe generalized junctional epidermolysis bullosa by systemic treatment with gentamicin. Dermatology. 2019;235:315–22.
Hao M, Antaya R, Cogan J, Hamilton C, Hou Y, Kwong A, et al. 861 Intravenous gentamicin therapy for junctional epidermolysis bullosa patients harboring nonsense mutations. J Investig Dermatol. 2020;140:S112.
Woodley D, Kwong A, Cogan J, Hou Y, Lincoln V, Mosallaei D, et al. 1021 Intravenous gentamicin therapy for recessive dystrophic epidermolysis bullosa patients harboring nonsense mutations. J Investig Dermatol. 2019;139:S176.
Welch EM, Barton ER, Zhuo J, Tomizawa Y, Friesen WJ, Trifillis P, et al. PTC124 targets genetic disorders caused by nonsense mutations. Nature. 2007;447:87–91.
McElroy SP, Nomura T, Torrie LS, Warbrick E, Gartner U, Wood G, et al. A lack of premature termination codon read-through efficacy of PTC124 (Ataluren) in a diverse array of reporter assays. PLoS Biol. 2013;11:e1001593.
Lincoln V, Cogan J, Hou Y, Hirsch M, Hao M, Alexeev V, et al. Gentamicin induces LAMB3 nonsense mutation readthrough and restores functional laminin 332 in junctional epidermolysis bullosa. Proc Natl Acad Sci USA. 2018;115:E6536–45.
Atanasova VS, Jiang Q, Prisco M, Gruber C, Pinon Hofbauer J, Chen M, et al. Amlexanox enhances premature termination codon read-through in COL7A1 and expression of full length type VII collagen: potential therapy for recessive dystrophic epidermolysis bullosa. J Investig Dermatol. 2017;137:1842–9.
Wally V, Lettner T, Peking P, Peckl-Schmid D, Murauer EM, Hainzl S, et al. The pathogenetic role of IL-1beta in severe epidermolysis bullosa simplex. J Investig Dermatol. 2013;133:1901–3.
Wally V, Hovnanian A, Ly J, Buckova H, Brunner V, Lettner T, et al. Diacerein orphan drug development for epidermolysis bullosa simplex: a phase 2/3 randomized, placebo-controlled, double-blind clinical trial. J Am Acad Dermatol. 2018;78:892–901.
Castela E, Tulic MK, Rozieres A, Bourrat E, Nicolas JF, Kanitakis J, et al. Epidermolysis bullosa simplex generalized severe induces a T helper 17 response and is improved by apremilast treatment. Br J Dermatol. 2019;180:357–64.
Odorisio T, Di Salvio M, Orecchia A, Di Zenzo G, Piccinni E, Cianfarani F, et al. Monozygotic twins discordant for recessive dystrophic epidermolysis bullosa phenotype highlight the role of TGF-β signalling in modifying disease severity. Hum Mol Genet. 2014;23:3907–22.
Lacro RV, Dietz HC, Sleeper LA, Yetman AT, Bradley TJ, Colan SD, et al. Atenolol versus losartan in children and young adults with Marfan’s syndrome. N Engl J Med. 2014;371:2061–71.
Nystrom A, Thriene K, Mittapalli V, Kern JS, Kiritsi D, Dengjel J, et al. Losartan ameliorates dystrophic epidermolysis bullosa and uncovers new disease mechanisms. EMBO Mol Med. 2015;7:1211–28.
.Kiritsi D. Losartan for RDEB trial—results and international perspectives. In: EB2020 1st world congress on epidermolysis bullosa, January 19–23, 2020, London, UK. Acta Derm Venereol. 2020;100.
Annicchiarico G, Morgese MG, Esposito S, Lopalco G, Lattarulo M, Tampoia M, et al. Proinflammatory cytokines and antiskin autoantibodies in patients with inherited epidermolysis bullosa. Medicine (Baltimore). 2015;94:e1528.
Gubinelli E, Angelo C, Pacifico V. A case of dystrophic epidermolysis bullosa improved with etanercept for concomitant psoriatic arthritis. Am J Clin Dermatol. 2010;11(Suppl 1):53–4.
Shehadeh W, Sarig O, Bar J, Sprecher E, Samuelov L. Treatment of epidermolysis bullosa pruriginosa-associated pruritus with dupilumab. Br J Dermatol. 2020;182:1495–7.
Zhou AG, Little AJ, Antaya RJ. Epidermolysis bullosa pruriginosa treated with dupilumab. Pediatr Dermatol. 2021;38:526–7.
Taha S, Al-Nesf M, Al-Obaidli A. Successful treatment of epidermolysis bullosa pruriginosa with anti-IgE therapy (Omalizumab) a case report and four years follow up. J Clin Exp Dermatol Res. 2020;11:520.
Tamai K, Yamazaki T, Chino T, Ishii M, Otsuru S, Kikuchi Y, et al. PDGFRalpha-positive cells in bone marrow are mobilized by high mobility group box 1 (HMGB1) to regenerate injured epithelia. Proc Natl Acad Sci USA. 2011;108:6609–14.
Aikawa E, Fujita R, Kikuchi Y, Kaneda Y, Tamai K. Systemic high-mobility group box 1 administration suppresses skin inflammation by inducing an accumulation of PDGFRalpha(+) mesenchymal cells from bone marrow. Sci Rep. 2015;5:11008.
Shimbo T, Yamazaki S, Wang X, Kikuchi Y, Bruckner-Tuderman L, Kaneda Y, et al. 906 Systemic HMGB1 administration ameliorates cutaneous and non-cutaneous manifestations in a dystrophic epidermolysis bullosa model mouse. J Investig Dermatol. 2017;137:S156.
Kido T, Miyagawa S, Goto T, Tamai K, Ueno T, Toda K, et al. The administration of high-mobility group box 1 fragment prevents deterioration of cardiac performance by enhancement of bone marrow mesenchymal stem cell homing in the delta-sarcoglycan-deficient hamster. PLoS ONE. 2018;13:e0202838.
Goto T, Miyagawa S, Tamai K, Matsuura R, Kido T, Kuratani T, et al. High-mobility group box 1 fragment suppresses adverse post-infarction remodeling by recruiting PDGFRalpha-positive bone marrow cells. PLoS ONE. 2020;15:e0230392.
Fine JD, Manes B, Frangoul H. Systemic granulocyte colony-stimulating factor (G-CSF) enhances wound healing in dystrophic epidermolysis bullosa (DEB): results of a pilot trial. J Am Acad Dermatol. 2015;73:56–61.
Yang WS, Kang S, Sung J, Kleinman HK. Thymosin beta4: potential to treat epidermolysis bullosa and other severe dermal injuries. Eur J Dermatol. 2019;29:459–67.
Fine JD. Epidermolysis bullosa: a genetic disease of altered cell adhesion and wound healing, and the possible clinical utility of topically applied thymosin beta4. Ann N Y Acad Sci. 2007;1112:396–406.
Goldstein AL, Kleinman HK. Advances in the basic and clinical applications of thymosin beta4. Expert Opin Biol Ther. 2015;15(Suppl 1):S139–45.
Kleinman HK, Sosne G. Thymosin beta4 promotes dermal healing. Vitam Horm. 2016;102:251–75.
Scheffler A. The wound healing properties of betulin from birch bark from bench to bedside. Planta Med. 2019;85:524–7.
Schwieger-Briel A, Ott H, Kiritsi D, Laszczyk-Lauer M, Bodemer C. Mechanism of Oleogel-S10: a triterpene preparation for the treatment of epidermolysis bullosa. Dermatol Ther. 2019;32:e12983.
Schwieger-Briel A, Kiritsi D, Schempp C, Has C, Schumann H. Betulin-based oleogel to improve wound healing in dystrophic epidermolysis bullosa: a prospective controlled proof-of-concept study. Dermatol Res Pract. 2017;2017:5068969.
Kern JS, Schwieger-Briel A, Lowe S, Sumeray M, Davis C, Martinez AE. Oleogel-S10 phase 3 study “EASE” for epidermolysis bullosa: study design and rationale. Trials. 2019;20:350.
Papanikolaou M, Onoufriadis A, Mellerio JE, Nattkemper LA, Yosipovitch G, Steinhoff M, et al. Prevalence, pathophysiology and management of itch in epidermolysis bullosa. Br J Dermatol. 2021;184:816–25.
Chiou AS, Choi S, Barriga M, Dutt-Singkh Y, Solis DC, Nazaroff J, et al. Phase 2 trial of a neurokinin-1 receptor antagonist for the treatment of chronic itch in patients with epidermolysis bullosa: a randomized clinical trial. J Am Acad Dermatol. 2020;82:1415–21.
Goldschneider KR, Good J, Harrop E, Liossi C, Lynch-Jordan A, Martinez AE, et al. Pain care for patients with epidermolysis bullosa: best care practice guidelines. BMC Med. 2014;12:178.
Schrader NHB, Duipmans JC, Molenbuur B, Wolff AP, Jonkman MF. Combined tetrahydrocannabinol and cannabidiol to treat pain in epidermolysis bullosa: a report of three cases. Br J Dermatol. 2019;180:922–4.
Moreno Artero E, Schinkel N, Chaumon S, Corset I, Rabeony T, Elie C, et al. Efficacy of topical ropivacaine in children and young adults with hereditary epidermolysis bullosa. Br J Dermatol. 2021;184:550–2.
Chelliah MP, Zinn Z, Khuu P, Teng JMC. Self-initiated use of topical cannabidiol oil for epidermolysis bullosa. Pediatr Dermatol. 2018;35:e224–7.
Abitbol RJ, Zhou LH. Treatment of epidermolysis bullosa simplex, Weber-Cockayne type, with botulinum toxin type A. Arch Dermatol. 2009;145:13–5.
Holahan HM, Farah RS, Ferguson NN, Paller AS, Legler AA. Treatment of symptomatic epidermolysis bullosa simplex with botulinum toxin in a pediatric patient. JAAD Case Rep. 2016;2:259–60.
Fine JD, Johnson LB, Weiner M, Li KP, Suchindran C. Epidermolysis bullosa and the risk of life-threatening cancers: the National EB Registry experience, 1986–2006. J Am Acad Dermatol. 2009;60:203–11.
Kim M, Li M, Intong LR, Tran K, Melbourne W, Marucci D, et al. Use of cetuximab as an adjuvant agent to radiotherapy and surgery in recessive dystrophic epidermolysis bullosa with squamous cell carcinoma. Br J Dermatol. 2013;169:208–10.
Diociaiuti A, Steinke H, Nystrom A, Schwieger-Briel A, Meiss F, Pfannenberg C, et al. EGFR inhibition for metastasized cutaneous squamous cell carcinoma in dystrophic epidermolysis bullosa. Orphanet J Rare Dis. 2019;14:278.
Medek K, Koelblinger P, Koller J, Diem A, Ude-Schoder K, Bauer JW, et al. Wound healing deficits in severe generalized recessive dystrophic epidermolysis bullosa along anticancer treatment with cetuximab. J Dtsch Dermatol Ges. 2019;17:448–50.
Arnold AW, Bruckner-Tuderman L, Zuger C, Itin PH. Cetuximab therapy of metastasizing cutaneous squamous cell carcinoma in a patient with severe recessive dystrophic epidermolysis bullosa. Dermatology. 2009;219:80–3.
Jalili A, Pinc A, Pieczkowski F, Karlhofer FM, Stingl G, Wagner SN. Combination of an EGFR blocker and a COX-2 inhibitor for the treatment of advanced cutaneous squamous cell carcinoma. J Dtsch Dermatol Ges. 2008;6:1066–9.
Reimer A, Lu S, He Y, Bruckner-Tuderman L, Technau-Hafsi K, Meiss F, et al. Combined anti-inflammatory and low-dose antiproliferative therapy for squamous cell carcinomas in recessive dystrophic epidermolysis bullosa. J Eur Acad Dermatol Venereol. 2020;34:e1–3.
Khaddour K, Gorell ES, Dehdashti F, Tang JY, Ansstas G. Induced remission of metastatic squamous cell carcinoma with an immune checkpoint inhibitor in a patient with recessive dystrophic epidermolysis bullosa. Case Rep Oncol. 2020;13:911–5.
Piccerillo A, El Hachem M, De Vito R, De Luca EV, Peris K. Pembrolizumab for treatment of a patient with multiple cutaneous squamous cell carcinomas and recessive dystrophic epidermolysis bullosa. JAMA Dermatol. 2020;156:708–10.
Watt SA, Pourreyron C, Purdie K, Hogan C, Cole CL, Foster N, et al. Integrative mRNA profiling comparing cultured primary cells with clinical samples reveals PLK1 and C20orf20 as therapeutic targets in cutaneous squamous cell carcinoma. Oncogene. 2011;30:4666–77.
Atanasova VS, Pourreyron C, Farshchian M, Lawler M, Brown CA 4th, Watt SA, et al. Identification of rigosertib for the treatment of recessive dystrophic epidermolysis bullosa-associated squamous cell carcinoma. Clin Cancer Res. 2019;25:3384–91.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No sources of funding were used to prepare this review.
Conflict of interest
The authors have no conflicts of interest that are directly relevant to the content of this review. The views expressed are those of the authors and not necessarily those of the UK National Health Service, the NIHR, or the UK Department of Health, or other health agencies.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Availability of data and material
Not applicable.
Code availability
Not applicable.
Author contributions
PCH, HTW, SA participated in literature review, manuscript creation, figure/table creation. WTT, JAM, CKH participated in manuscript revision, figure/table revision.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hou, PC., Wang, HT., Abhee, S. et al. Investigational Treatments for Epidermolysis Bullosa. Am J Clin Dermatol 22, 801–817 (2021). https://doi.org/10.1007/s40257-021-00626-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40257-021-00626-3