In biochemistry, DNA holds the genetic code for life. When genes are expressed, the DNA is transcribed into mRNA that is then translated into proteins. Proteins are complex biomolecules that perform critical roles in the cell. These proteins are made up of smaller building blocks called amino acids (AA). The AAs are strung together by ribosomes that read the instructions given to it by the mRNA. Errors in amino acid placement do occur and can lead to cell death in some instances. Always keep in mind, structure gives function.
Study Tips
Most biochemistry courses will require you to know the following: the amino acid name, the structure, the pKa of ionizable hydrogens, and both the 3-letter and 1-letter shorthand. That is a daunting task for 20 amino acids.
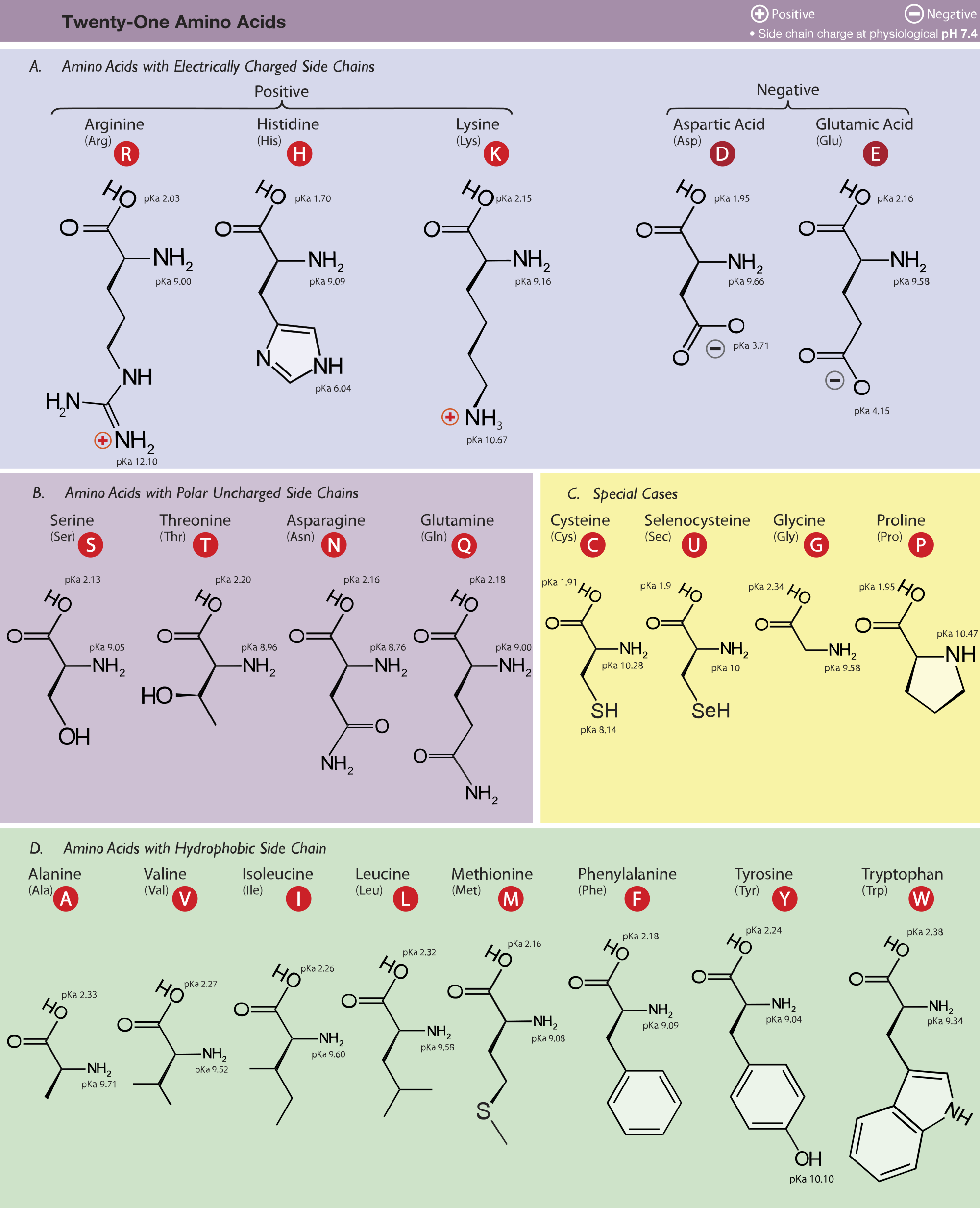
To learn the structures, names, and shorthand, the best method here is memorization. Use flash cards, whiteboards, or any other method of repetitive memorization. A table of the common amino acids is provided in Figure 1. This should be downloaded and printed. Note that selenocysteine (Sec, U) is not a common amino acid and can be skipped.
To read Figure 1, the AA in the top left is arginine. The three letters in parentheses are the 3-letter shorthand, and the letter in the red circle is the 1-letter shorthand: e.g., arginine, Arg, R. Keep the table with you; like the periodic table of elements in chemistry, you will refer to this AA table throughout the course. Also be keen on the nomenclature and conventions presented to you in class.
Memorization only goes so far. To learn pH effects or the physicochemical properties of individual AAs, it’s best to try to understand the process and how changing the microenvironment will alter how an AA will behave. Also, some amino acids have unique traits that add to their functionality
Carbon Skeleton
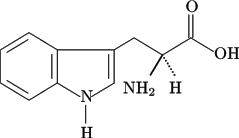
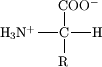
All of the amino acids are composed of an amine(-NH2) and a carboxylicacid (-COOH) linked by a central a-carbon (Figure 2). The R group, or side chain, is unique for each amino acid; as a result, the a-carbon is a chiral center, except for glycine where R = hydrogen. Biochemists focus on L-amino acids, as depicted in Figure 1 (wedge bonds), but D-amino acids are relevant for pharmaceutical applications.
If you are confused about stereochemistry, refresh yourself on Fischer projections from organic chemistry. Course instructors will draw structures many different ways. Ask the instructor to provide the stereochemistry whenever possible. Reversing or inverting the structure will have implications on the wedge or hashed (dashed) marked bonds (Figure 3).
Amino acids are the monomeric building blocks of proteins;the cell builds proteins by joining amino acids to each other from carboxylic group to amine group. The newly formed amide bond or peptide bond is formed by a dehydration reaction. Short sequences less than 50 AAs are called peptides and sequences greater than 50 AAs are referred to as proteins. In literature, a particular AA may be referred to as a residue; e.g., “The lysine residues in histones are acetylated to promote dsDNA packaging.”
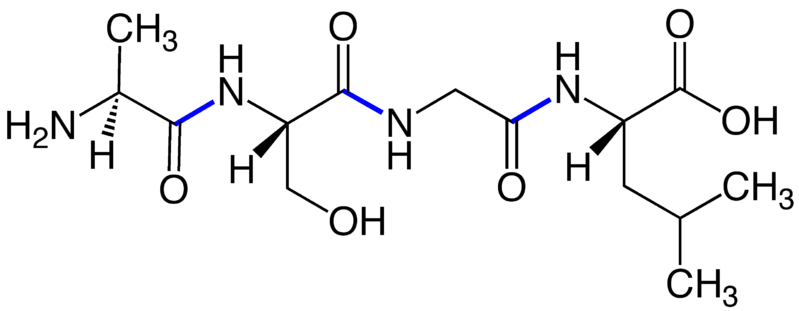
In Figure 3, four amino acids–alanine, serine, glycine and leucine–are connected by peptide bonds (blue). The –NH2 group of the first peptide is called the amine orN-terminus; the –COOH group of the last peptide is the carboxy orC-terminus. Peptides and proteins are written and numbered left to right, starting at the N-terminus and ending at the C-terminus. The sequence above (in 3-letter code) is Ala1-Ser2-Gly3-Leu4. In the cell, proteins are synthesized from the amine terminus to the carboxy terminus, and we maintain this convention in reporting sequences. Errors in synthesis (mutations) are reported like this: Ser2 ➔ Thr; this means the serine in position 2 was mutated to a threonine. In 1-letter shorthand: S2T.
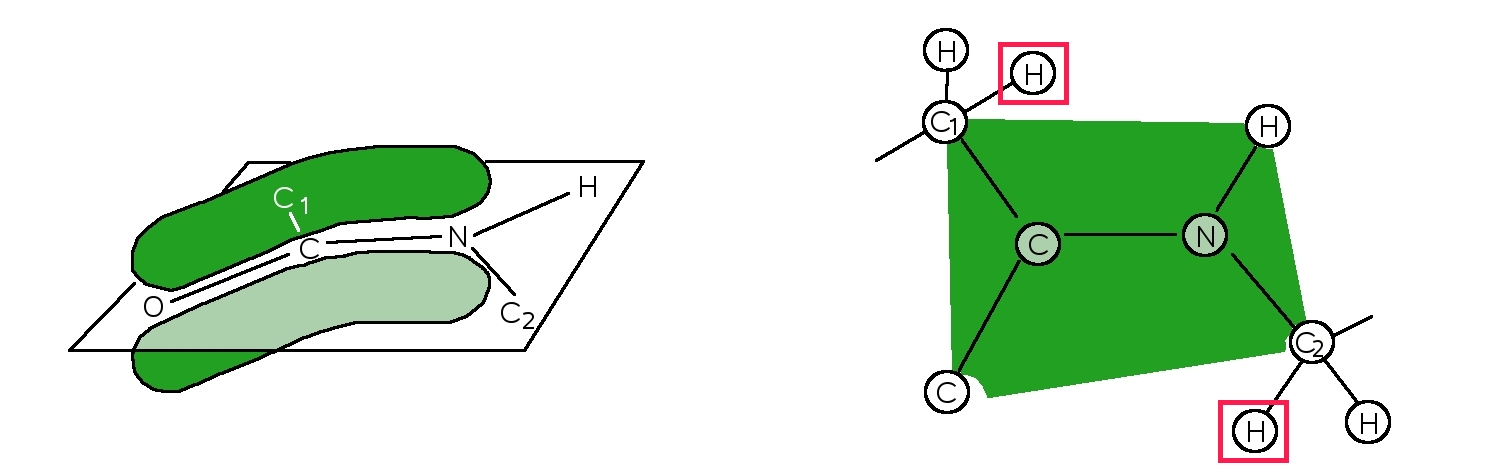
When AAs are joined, the resulting peptide bond has a planar geometry (Figure 4). The alignment of the p-orbitals between the amide nitrogen and the carbonyl carbon and oxygen facilitates the sharing of electrons, strengthening the bonds, making them rigid pseudo-double bonds. This is known as tautomerization. The a-carbons on either side are locked into specific geometries that will later form a-helices and b-sheets in proteins.
Amino Acid Classification
The 20 common amino acids can be classified by their side chains. The two main groups are the hydrophobic amino acids(water fearing) and the hydrophilic amino acids(water loving). The ever present solvent in cells is water; physiological conditions assume a pH of 7.4 and temperature of approximately 37 °C. Note that pH is unit-less and there is a space before the degree symbol.
The hydrophobic-amino acids are non-polar, and limit their exposure to water. These residues tend to bury themselves towards the cores of proteins. Hydrophobic amino acids can be further divided into alkyl or aromaticresidues. The alkyl side chains resemble saturated hydrocarbon chains and include glycine, alanine, valine, leucine, isoleucine, methionine, and proline. The aromatic amino acids are phenylalanine and tryptophan. The aromatics can self-stabilize by p-stacking on each other (like pancakes).
The hydrophilic amino acids are polar and will gravitate towards any surface exposed to water. Outer surfaces of proteins and protein channels are lined with these residues. Hydrophilic amino acids can be broken down into three groups: neutral, acidic, and basic amino acids. The neutral amino acids are tyrosine, serine, threonine, cysteine, glutamine, and asparagine. Note that tyrosine is both polar and aromatic. The acidic amino acids are glutamic acid (glutamate) and aspartic acid (aspartate). The basic amino acids are lysine, histidine, and arginine. A full discussion of pH effects for each of these AA types is down below.
Summary of Amino Acids
This table summarizes the properties of AAs and will provide tips for quick identification. Refer to Figure 1 for full structures of L-amino acids.
| Name | 3-Letter code | 1-Letter code | Side chain | |
| Glycine | Gly | G | H | Optically inactive. |
| Alanine | Ala | A | -CH3 | The simplest optically active AA. All other AAs will build off alanine, A like the beginning of the alphabet. |
| Valine | Val | V | -CH(CH3)2 | The side chain is branched like a V. |
| Leucine | Leu | L | -CH2[CH(CH3)2] | The side chain is branched like a Y. |
| Isoleucine | Ile | I | -CH(CH3)(C2H5) | An isomer of leucine. One branch is longer than the other. |
| Proline | Pro | P | -CH2 CH2CH2– | The only cyclic aliphatic. |
| Cysteine | Cys | C | -CH2-SH | Cysteine has a sulfhydryl (SH) group. |
| Methionine | Met | M | -(CH2)-S-(CH3) | Thiol is synonymous with sulfur. Methionine is a methylated sulfur. |
| Phenylalanine | Phe | F | -CH2(C6H5) | A benzene (or phenyl) ring attached to an alanine. F like its name. |
| Tryptophan | Trp | W | -indole ring | AA with the largest side chain, two fused rings (double). |
| Tyrosine | Tyr | Y | -CH2(C6H4OH) | A hydroxylated (OH) phenylalanine. |
| Serine | Ser | S | -CH2OH | A hydroxylated alanine. |
| Threonine | Thr | T | – CH(OH)(CH3) | Three parts to this AA: alanine, methyl, and hydroxyl. |
| Arginine | Arg | R | -(CH2)3-urea | The side chain has a urea (NH2-C (NH2+)(NH)) group (A pirates favorite AA, arginine). |
| Lysine | Lys | K | -(CH2)4-NH3+ | The basic straight chain AA with amino group . |
| Histidine | His | H | -CH2-(C3N2H4)+ | This amino acid has an imidazole, an aromatic ring with two nitrogen atoms that can be protonated. |
| Aspartate | Asp | D | -CH2COOH | An acidic alanine. Just remember D. |
| Glutamate | Glu | E | -CH2CH2COOH | A longer version of Asp. E follows D in the alphabet. |
| Asparagine | Asn | N | -CH2CONH2 | Amidated Asp. |
| Glutamine | Gln | Q | -CH2CH2CONH2 | AmidatedGlu. |
Special Properties of Amino Acids
Some amino acids have unique properties. These properties can affect the final protein structure, the electrostatics of the protein, or aid in quantification of protein concentrations.
Cysteine
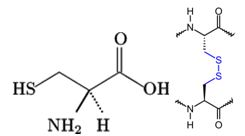
Figure 5 shows a cysteine as a free thiol (-SH). The thiol group is very reactive to oxidation reactions and will form a disulfide bridge with another cysteine. This bond can form between cysteines on the same polypeptide chain (intramolecular) or between two different strands (intermolecular). This covalent bond locks the peptide backbone into a specific orientation and can only be broken under reducing conditions. Methionine, the other sulfur-containing AA, cannot form disulfide bridges and is the start codon for protein synthesis.
Proline and Glycine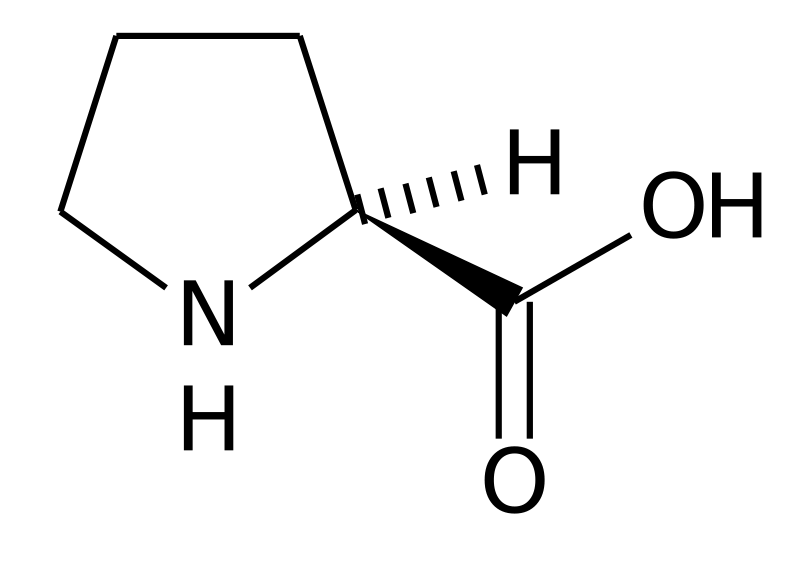
Figure 6.Proline (top) and glycine (bottom). Image Source: Wikimedia Commons
Structurally, proline (Figure 6) is unique among the AAs because its side chain loops around and reconnects with the peptide backbone. All the other AAs have primary amines, and when bound in a polypeptide chain, they become secondary amides; Pro within the chain becomes a tertiary amide. Due to Pro locked geometry, kinks are introduced into the peptide chain. Glycine, on the other hand, has no side chain. There is a lot of free rotation about the a-carbon; these points are very flexible in the peptide chain. Proline and glycine work together to disrupt the secondary structure of proteins and are known as a-helix breakers.
Tyrosine and Tryptophan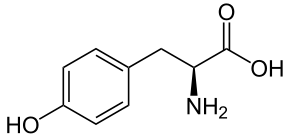
Figure 7. Tyrosine (left) and tryptophan (right). Image Source: Wikimedia Commons
Tyrosine and tryptophan are two of the aromatic amino acids (Figure 7) and exhibit strong UV-light absorption at 280 nm. Phenylalanine, also aromatic, absorbs at a much lower frequency. Proteins and peptides that contain either Tyr or Trp can be quantified by UV-Vis spectroscopy because they absorb light in the UV light spectrum. The molar extinction coefficients are Tyr = 1490 L cm-1M-1and Trp = 5500 L cm-1M-1. These coefficients are additive; for example, if you have a protein with two Tyr and three Trp, that protein’s extinction coefficient = (2 x 1490) + (3 x 5500) = 19,480L cm-1M-1.
Histidine
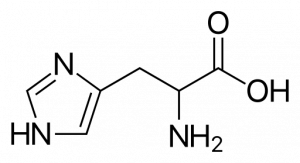
Histidine has an imidazole side chain (Figure 8) that has a pKa of 6.0, which is close to the physiological pH of 7.4. This allows His to act as a buffer that can accept or donate hydrogens when needed. Many active sites employ His to mediate reactions. His can also be used structurally in a salt bridge.
Acids, Bases, and Salt Bridges
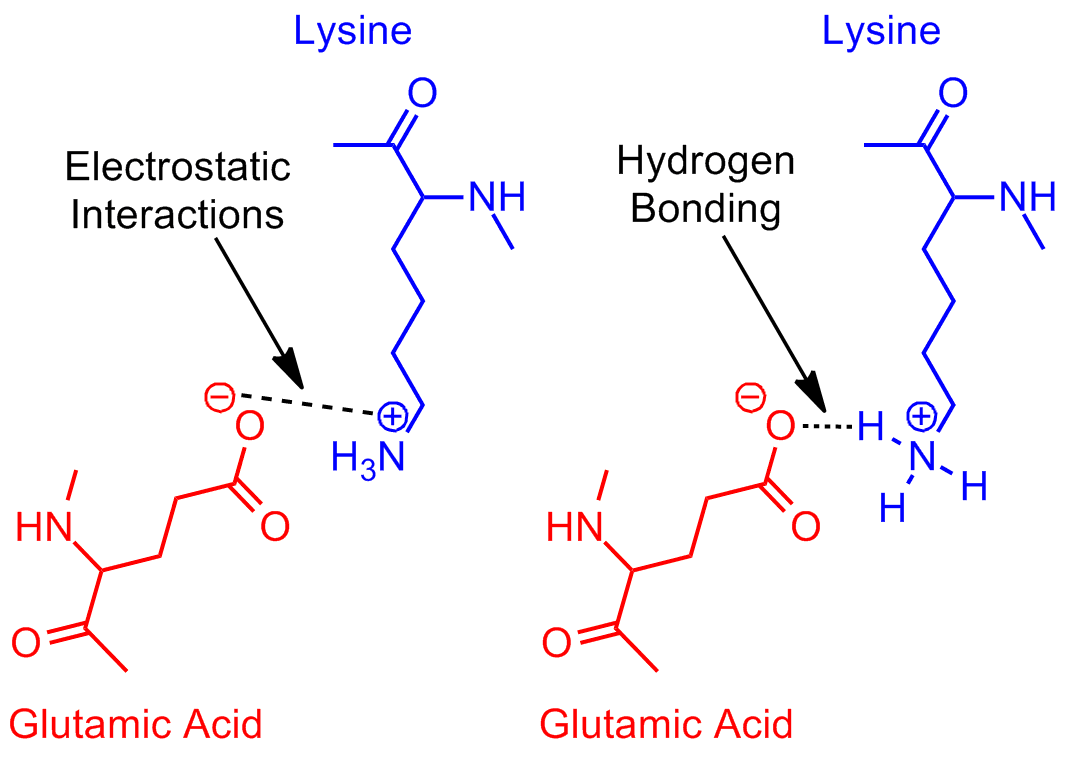
The last groups are the acids–aspartate and glutamate–and bases—arginine, lysine and sometimes histidine–that form salt bridges (Figure 9). The electrostatic interactions between positive bases and negative acids through space stabilize protein structures. These interactions are stronger than hydrogen bonds (4 kcal/mol), but weaker than disulfide bonds (60 kcal/mol). Salt bridges are also found in the binding sites of proteins, often holding ligands for transport or substrates for enzymatic reactions.
Effects of pH
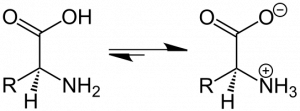
Amino acids have both a basic motif and an acidic motif. At physiological pH most AAs will be zwitterions (Figure 10), not considering the side chains. The carboxy terminus is deprotonated with a negative charge while the amine terminus is protonated with a positive charge. The net charge on the molecule is zero. By altering the pH of the system, you can protonate or deprotonate the termini. When the AAs form peptide bonds, they no longer contribute to pH effects.
Non-ionizable Side Chains
Most of the amino acids do not have ionizable side chains, and you only have to concern yourself with protonation of the termini when studying pH effects: this applies to Gly, Ala, Val, Leu, Ile, Met, Pro, Phe, Thr, Ser, Asn, Gln, and Trp. The average pKa of the C-terminus is ~2.0, meaning that if the pH is greater than 2, it is in the carboxylate (-COO–) form, and if the pH is less than 2, it is in the protonated form (-COOH). For the N-terminus, the average pKa is ~9.5. When the pH is greater than 9.5, the amine is deprotonated (-NH3) until the pH drops below 9.5, then the protonated amine is formed (-NH4+). The key ideas are (1) the lower the pH, the more hydrogen (H+) present and (2) bases will protonate before acids.
Ionizable Side Chains
For the amino acids Arg, Lys, Tyr, His, Cys, Asp, and Glu you must take into account side chains. Figure 1 lists side chain pKa’s. The bases, Arg and Lys, have side chain pKa’s of 12.3 and 10.7 respectively. At physiological conditions, they are protonated. The pKa of the acidic AAs are 3.7 for Asp and 4.15 for Glu. They are deprotonated in the cell. The buffering capacity of His was explained earlier; its pKa is more acidic at 6.0.
Tyr and Cys behave like alcohols, but unlike Ser and Thr, they can be deprotonated easily. Cys would prefer to bind with another Cys, but free thiolCys does occur in proteins, sometimes in active sites. Its pKa is 8.37. Tyr has a pKa at 10.1, similar to Lys due to resonance stabilization.
Isoelectric Focusing and Electrophoresis
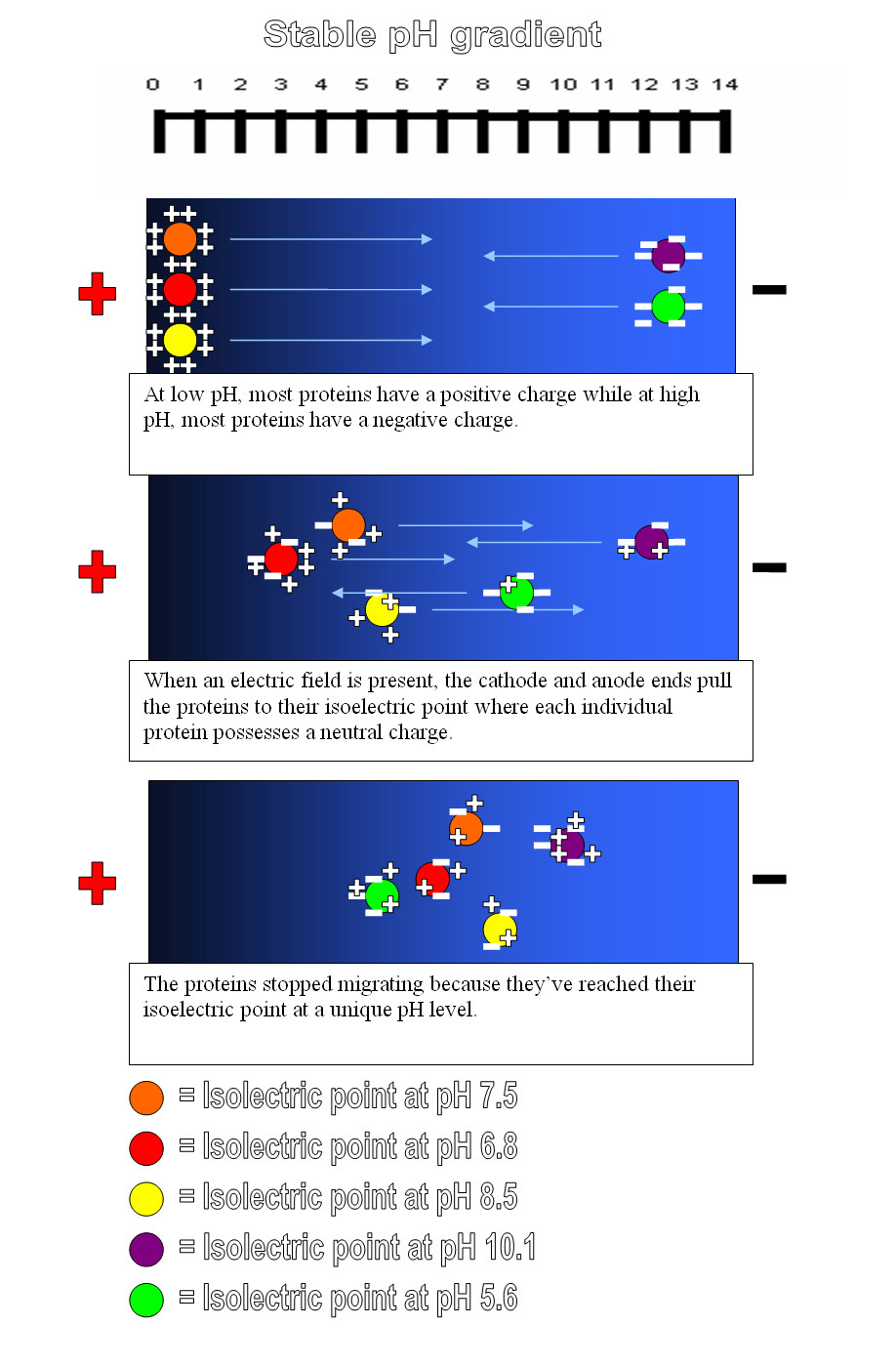
What is the importance of charge on an amino acid? It turns out that amino acids, and by extension proteins, can be manipulated based on their charge. The isoelectric point (pI) is the pH at which amino acids or proteins have a net zero charge. A mixed sample of AAs can then be separated by isoelectric focusing (IEF) using a pH gradient and an electric current (Figure 11).
The movement of charged particles relative to a fluid in a uniform electric field is called electrophoresis. An extension of IEF is Western Blotting, where samples are first separated by electrophoresis, then transferred to a binding medium and assayed.
Post-Translational Modifications
After the ribosome has finished processing the mRNA, certain amino acids can be enzymatically modified. These are known as post-translational modifications (PTM). The PTM occur on specific AAs or on a residue in a specific sequence.
Acetylationcan occur on the side chain of lysine. The addition of ubiquitin, a protein, is done by acetylation. Ubiquitination is important for ubiquitin-mediated protein degradation, a method for flagging proteins for recycling. Other common types of acetylation are lipidations and prenylations on cysteine and N-terminal glycines.
Phosphorylation adds a -OPO3-2 to serine, threonine, tyrosine, histidine, arginine, or lysine. This type of PTM dramatically changes the electrostatics of the residue; neutral or basic AAs gain a -2 charge. Cells use phosphorylation as a method of signal transduction to activate or deactivate metabolic pathways.
Glycosylation adds a carbohydrate to an AA. Reported as Asn-X-Ser or Asn-X-Thr, where X is any residue, this consensus sequence means that glycosylation will occur at a serine or threonine that is two residues away from an asparagine.
Summary
It is a lot to take in, so do it in parts. Memorize the amino acid names and structures as quickly as possible, but take the time to understand the physical processes that affect amino acids. These small changes will have a huge impact on the structure and function of proteins.
Was this article helpful? We would like to hear from you. What study tip helps you the most?
Let’s put everything into practice. Try this Biochemistry practice question:
Looking for more Biochemistry practice?
You can find thousands of practice questions on Albert.io. Albert.io lets you customize your learning experience to target practice where you need the most help. We’ll give you challenging practice questions to help you achieve mastery in Biochemistry.
Start practicing here.
Are you a teacher or administrator interested in boosting Biochemistry student outcomes?
Learn more about our school licenses here.