Prospects of Hogweed (Heracleum sphondylium L.) as a New Horticultural Crop for Food and Non-Food Uses: A Review
Abstract
:1. Introduction
2. Methodology
2.1. Step 1: Selecting Research Questions, Databases, Websites, and Appropriate Search Terms
2.2. Step 2: Applying Practical Screening Criteria
2.3. Step 3: Applying Methodological Screening Criteria
3. Species Description
3.1. Distribution and Habitat
3.2. Morphology and Biology
4. Ethnobotanical Knowledge
5. Food Uses
6. Phytochemistry and Biological Activity
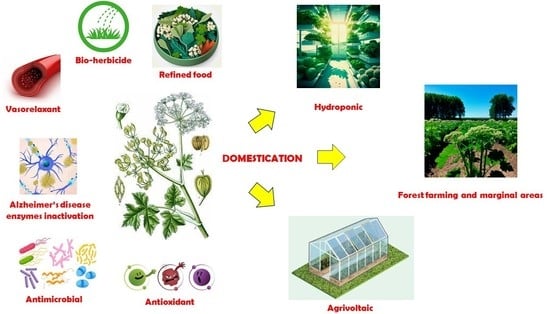
7. Domestication
8. Prospect
9. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Petropoulos, S.A.; Karkanis, A.; Martins, N.; Ferreira, I.C.F.R. Edible halophytes of the Mediterranean basin: Potential candidates for novel food products. Trends Food Sci. Technol. 2018, 74, 69–84. [Google Scholar] [CrossRef]
- Licata, M.; Tuttolomondo, T.; Leto, C.; Virga, G.; Bonsangue, G.; Cammalleri, I.; Gennaro, M.C.; La Bella, S. A survey of wild plant species for food use in Sicily (Italy)—Results of a 3-year study in four Regional Parks. J. Ethnobiol. Ethnomed. 2016, 12. [Google Scholar] [CrossRef]
- Geraci, A.; Amato, F.; Di Noto, G.; Bazan, G.; Schicchi, R. The wild taxa utilized as vegetables in Sicily (Italy): A traditional component of the Mediterranean diet. J. Ethnobiol. Ethnomed. 2018, 14, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Lentini, F.; Venza, F. Wild food plants of popular use in Sicily. J. Ethnobiol. Ethnomed. 2007, 3, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Mattirolo, O.; Gallino, B.; Pallavicini, G. Phytoalimurgia Pedemontana.; Blu edizioni: Pevegnano, Italy, 2011; ISBN 9788879041218. [Google Scholar]
- Elia, A.; Santamaria, P. Biodiversity in vegetable crops, a heritage to save: The case of Puglia region. Ital. J. Agron. 2013, 8, 4. [Google Scholar] [CrossRef]
- Pieroni, A.; Nebel, S.; Santoro, R.F.; Heinrich, M. Food for two seasons: Culinary uses of non-cultivated local vegetables and mushrooms in a south Italian village. Int. J. Food Sci. Nutr. 2005, 56, 245–272. [Google Scholar] [CrossRef]
- Morales, P.; Ferreira, I.C.F.R.; Carvalho, A.M.; Sánchez-Mata, M.C.; Cámara, M.; Fernández-Ruiz, V.; Pardo-de-Santayana, M.; Tardío, J. Mediterranean non-cultivated vegetables as dietary sources of compounds with antioxidant and biological activity. LWT Food Sci. Technol. 2014, 55, 389–396. [Google Scholar] [CrossRef]
- Benincasa, P.; Tei, F.; Rosati, A. Plant density and genotype effects on wild asparagus (Asparagus acutifolius L.) spear yield and quality. HortScience 2007, 42, 1163–1166. [Google Scholar] [CrossRef]
- Dorais, M.; Papadopoulos, A.P.; Luo, X.; Leonhart, S.; Gosselin, A.; Pedneault, K.; Angers, P.; Gaudreau, L. Soilless greenhouse production of medicinal plants in north Eastern Canada. Acta Hortic. 2001, 554, 297–303. [Google Scholar] [CrossRef]
- Bonasia, A.; Conversa, G.; Lazzizera, C.; La Rotonda, P.; Elia, A. Weed control in lampascione - Muscari comosum (L.) Mill. Crop Prot. 2012, 36, 65–72. [Google Scholar] [CrossRef]
- Branca, F.; Fisichella, A. Response of Brassica fruticulosa Cyr. to greenhouse cultivation. In Proceedings of the VI International Symposium on Protected Cultivation in Mild Winter Climate: Product and Process Innovation 614, Ragusa, Sicily, 8 March 2003; Volume 614, pp. 89–93. [Google Scholar]
- Senejoux, F.; Demougeot, C.; Cuciureanu, M.; Miron, A.; Cuciureanu, R.; Berthelot, A.; Girard-Thernier, C. Vasorelaxant effects and mechanisms of action of Heracleum sphondylium L. (Apiaceae) in rat thoracic aorta. J. Ethnopharmacol. 2013, 147, 536–539. [Google Scholar] [CrossRef] [PubMed]
- Heracleum sphondylium L.—USDA Plants Database. Available online: https://plants.sc.egov.usda.gov/home/plantProfile?symbol=HESP6 (accessed on 24 December 2022).
- Borscht. Available online: https://en.wikipedia.org/wiki/Borscht (accessed on 25 January 2023).
- Işcan, G.; Demirci, F.; Kürkçüoǧlu, M.; Kivanç, M.; Başer, K.H.C. The bioactive essential oil of Heracleum sphondylium L. subsp. ternatum (Velen.) Brummitt. Zeitschrift fur Naturforsch. Sect. C J. Biosci. 2003, 58, 195–200. [Google Scholar] [CrossRef]
- Uysal, A.; Ozer, O.Y.; Zengin, G.; Stefanucci, A.; Mollica, A.; Picot-Allain, C.M.N.; Mahomoodally, M.F. Multifunctional approaches to provide potential pharmacophores for the pharmacy shelf: Heracleum sphondylium L. subsp. ternatum (Velen.) Brummitt. Comput. Biol. Chem. 2019, 78, 64–73. [Google Scholar] [CrossRef]
- Tranfield, D.; Denyer, D.; Smart, P. Towards a Methodology for Developing Evidence-Informed Management Knowledge by Means of Systematic Review. Br. J. Manag. 2003, 14, 207–222. [Google Scholar] [CrossRef]
- Heracleum sphondylium. Available online: https://en.wikipedia.org/wiki/Heracleum_sphondylium (accessed on 26 January 2023).
- Heracleum sphondylium: Sistematica, Etimologia, Habitat, Coltivazione. Available online: https://antropocene.it/2021/05/08/heracleum-sphondylium/ (accessed on 27 December 2022).
- Devillers, P.; Devillers-Tcrschuren, J.; Ledant, J.-P. CORINE Biotopes Manual. Habitats of the European Community. Data Specifications—Part 2.; Office for Official Publications of the European Communities: Luxemburg, 1991; ISBN 92-826-3228-8. [Google Scholar]
- Sheppard, A.W. Heracleum sphondylium L. J. Ecol. 1991, 79, 235–258. [Google Scholar] [CrossRef]
- Dominik, T.; Pachlewski, R. Badanie mykotrofizmu zespolow roslinnych regla dolnego w Tatrac. Acta Soc. Bot. Corum Pol. 1956, 25, 3–26. [Google Scholar] [CrossRef]
- Common Hogweed—Identification, Edibility, Distribution—Galloway Wild Foods. Available online: https://gallowaywildfoods.com/hogweed/ (accessed on 24 December 2022).
- Euleia heracleid. Available online: https://www.eakringbirds.com/eakringbirds4/insectinfocuseuleiaheraclei.htm (accessed on 24 December 2022).
- Anderson, H.A. Berries: A Global History; Reaktion Books: New York, NY, USA; Dover, DE, USA, 2018; ISBN 9781780239385. [Google Scholar]
- Colombo, M.L.; Luciano, R. Ombrellifere Della Provincia di Cuneo; ArabaFenice, 2008; ISBN 9788895853000. Available online: https://www.amazon.co.uk/Ombrellifere-della-provincia-Cuneo-Colombo/dp/8895853008 (accessed on 29 January 2023).
- Bussmann, R.W. Ethnobotany of the Caucasus; Bussmann, R.W., Ed.; Springer: Cham, Switzerland, 2017; ISBN 9783-319494111. [Google Scholar]
- Renna, M.; Gonnella, M. The use of the sea fennel as a new spice-colorant in culinary preparations. Int. J. Gastron. Food Sci. 2012, 1, 111–115. [Google Scholar] [CrossRef]
- Renna, M.; Gonnella, M.; Caretto, S.; Mita, G.; Serio, F. Sea fennel (Crithmum maritimum L.): From underutilized crop to new dried product for food use. Genet. Resour. Crop Evol. 2017, 64, 205–216. [Google Scholar] [CrossRef]
- Bicchi, C.; D’Amato, A.; Frattini, C.; Cappelletti, E.M.; Caniato, R.; Filippini, R. Chemical diversity of the contents from the secretory structures of Heracleum sphondylium subsp. sphondylium. Phytochemistry 1990, 29, 1883–1887. [Google Scholar] [CrossRef]
- Muckensturm, B.; Duplay, D.; Robert, P.C.; Simonis, M.T.; Kienlen, J.C. Substances antiappétantes pour insectes phytophages présentes dans Angelica sylvestris et Heracleum sphondylium. Biochem. Syst. Ecol. 1981, 9, 289–292. [Google Scholar] [CrossRef]
- Brazdovicova, B.; Kostalova, D.; Strocinska, E.; Tomko, J. Isolation and identification of oroselol and other furocoumarin derivatives from Heracleum sphondylium L. roots. Cesk. Farm. 1982, 31, 346–347. [Google Scholar] [PubMed]
- Maggi, F.; Quassinti, L.; Bramucci, M.; Lupidi, G.; Petrelli, D.; Vitali, L.A.; Papa, F.; Vittori, S. Composition and biological activities of hogweed [Heracleum sphondylium L. subsp. ternatum (Velen.) Brummitt] essential oil and its main components octyl acetate and octyl butyrate. Nat. Prod. Res. 2014, 28, 1354–1363. [Google Scholar] [CrossRef]
- Melough, M.M.; Cho, E.; Chun, O.K. Furocoumarins: A review of biochemical activities, dietary sources and intake, and potential health risks. Food Chem. Toxicol. 2018, 113, 99–107. [Google Scholar] [CrossRef]
- Paathak, M.A.; Farrington, D.; Fitzpatrick, T.B. The presently known distribution of furocoumarins (psoralens) in plants. J. Investig. Dermatol. 1962, 39, 225–239. [Google Scholar] [CrossRef] [PubMed]
- Bahadori, M.B.; Dinparast, L.; Zengin, G. The Genus Heracleum: A Comprehensive Review on Its Phytochemistry, Pharmacology, and Ethnobotanical Values as a Useful Herb. Compr. Rev. Food Sci. Food Saf. 2016, 15, 1018–1039. [Google Scholar] [CrossRef] [PubMed]
- Iscan, G.; Ozek, T.; Ozek, G.; Duran, A.; Baser, K.H.C. Essential oils of three species of Heracleum. Anticandidal activity. Chem. Nat. Compd. 2004, 40, 544–547. [Google Scholar] [CrossRef]
- Fierascu, R.C.; Padure, I.M.; Avramescu, S.M.; Ungureanu, C.; Bunghez, R.I.; Ortan, A.; Dinu-Pirvu, C.; Fierascu, I.; Soare, L.C. Preliminary assessment of the antioxidant, antifungal and germination inhibitory potential of Heracleum sphondylium L. (Apiaceae). Farmacia 2016, 64, 403–408. [Google Scholar]
- Benedec, D.; Hanganu, D.; Filip, L.; Oniga, I.; Tiperciuc, B.; Olah, N.K.; Gheldiu, A.M.; Raita, O.; Vlase, L. Chemical, antioxidant and antibacterial studies of Romanian Heracleum sphondylium. Farmacia 2017, 65, 252–256. [Google Scholar]
- Baskin, C.C.; Baskin, J.M. Seeds: Ecology, Biogeography, and Evolution of Dormancy and Germination; Academic Press Inc.: San Diego, CA, USA, 1998; ISBN 978-0-12-080260-9. [Google Scholar]
- Stokes, P. A Physiological Study of Embryo Development in Heracleum sphondylium L. Ann. Bot. 1952, 16, 441–447. [Google Scholar] [CrossRef]
- Luna, T. Propagation Protocol for Production of Container (Plug) Heracleum maximum Bartr. Plants 172 mL Conetainers. Available online: https://npn.rngr.net/renderNPNProtocolDetails?selectedProtocolIds=apiaceae-heracleum-5 (accessed on 29 January 2023).
- Nowruzian, A.; Masoumian, M.; Ebrahimi, M.A.; Bakhshi Khaniki, G.R. Effect of Breaking Dormancy Treatments on Germination of Ferula assa-foetida Seed. Iran. J. Seed Res. 2017, 3, 155–169. [Google Scholar] [CrossRef]
- Renna, M. Reviewing the prospects of sea fennel (Crithmum maritimum L.) as emerging vegetable crop. Plants 2018, 7, 92. [Google Scholar] [CrossRef]
- William, E. Botanical Composition of the Park Grass Plots at Rothamsted 1856–1976; Rothamsted Experimental Station: Hertfordshire, UK, 1978. [Google Scholar]
- Fracchiolla, M.; Renna, M.; D’Imperio, M.; Lasorella, C.; Santamaria, P.; Cazzato, E. Living Mulch and Organic Fertilization to Improve Weed Management, Yield and Quality of Broccoli Raab in Organic Farming. Plants 2020, 9, 177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fracchiolla, M.; Renna, M.; Durante, M.; Mita, G.; Serio, F.; Cazzato, E. Cover crops and manure combined with commercial fertilizers differently affect yield and quality of processing tomato (Solanum lycopersicum L.) organically grown in puglia. Agriculture 2021, 11, 757. [Google Scholar] [CrossRef]
- Mudge, K.; Gabriel, S. Farming the Woods: An Integrated Permaculture Approach to Growing Food and Medicinals in Temperate Forests; Chelsea Green Publishing: White River Junction, VT, USA, 2014. [Google Scholar]
- Bin, Y.W.; Hernandez, J.O.; Park, B.B. Effects of shade and planting methods on the growth of Heracleum moellendorffii and adenophora divaricata in different soil moisture and nutrient conditions. Plants 2021, 10, 2203. [Google Scholar] [CrossRef]
- Henry, P.; Provan, J.; Goudet, J.; Guisan, A.; Jahodová, Š.; Besnard, G. A set of primers for plastid indels and nuclear microsatellites in the invasive plant Heracleum mantegazzianum (Apiaceae) and their transferability to Heracleum sphondylium. Mol. Ecol. Resour. 2008, 8, 161–163. [Google Scholar] [CrossRef] [PubMed]
- Grzędzicka, E. Invasion of the Giant Hogweed and the Sosnowsky’s Hogweed as a Multidisciplinary Problem with Unknown Future—A Review. Earth 2022, 3, 287–312. [Google Scholar] [CrossRef]
- Niinikoski, P.; Korpelainen, H. Population genetics of the invasive giant hogweed (Heracleum sp.) in a northern European region. Plant Ecol. 2015, 216, 1155–1162. [Google Scholar] [CrossRef]
- Anaclerio, M.; Renna, M.; di Venere, D.; Sergio, L.; Santamaria, P. Smooth golden fleece and prickly golden fleece as potential new vegetables for the ready-to-eat production chain. Agriculture 2021, 11, 74. [Google Scholar] [CrossRef]
- Montesano, F.F.; Gattullo, C.E.; Parente, A.; Terzano, R.; Renna, M. Cultivation of Potted Sea Fennel, an Emerging Mediterranean Halophyte, Using a Renewable Seaweed-Based Material as a Peat Substitute. Agriculture 2018, 8, 96. [Google Scholar] [CrossRef]
- Gonnella, M.; Renna, M. The evolution of soilless systems towards ecological sustainability in the perspective of a circular economy. Is it really the opposite of organic agriculture? Agronomy 2021, 11, 950. [Google Scholar] [CrossRef]
- D’Imperio, M.; Parente, A.; Montesano, F.F.; Renna, M.; Logrieco, A.F.; Serio, F. Boron Biofortification of Portulaca oleracea L. through Soilless Cultivation for a New Tailored Crop. Agronomy 2020, 10, 999. [Google Scholar] [CrossRef]
- D’Imperio, M.; Durante, M.; Gonnella, M.; Renna, M.; Montesano, F.F.; Parente, A.; Mita, G.; Serio, F. Enhancing the nutritional value of Portulaca oleracea L. by using soilless agronomic biofortification with zinc. Food Res. Int. 2022, 155, 111057. [Google Scholar] [CrossRef] [PubMed]
- D’Imperio, M.; Renna, M.; Cardinali, A.; Buttaro, D.; Santamaria, P.; Serio, F. Silicon biofortification of leafy vegetables and its bioaccessibility in the edible parts. J. Sci. Food Agric. 2016, 96, 751–756. [Google Scholar] [CrossRef]
- Puccinelli, M.; Pezzarossa, B.; Pintimalli, L.; Malorgio, F. Selenium biofortification of three wild species, Rumex acetosa L., Plantago coronopus L., and Portulaca oleracea L., grown as microgreens. Agronomy 2021, 11, 1155. [Google Scholar] [CrossRef]
- Nascimento, C.S.; Nascimento, C.S.; Lopes, G.; Carrasco, G.; Lupino, P.; Bernardes, A. Biofortified Rocket (Eruca sativa) with Selenium by Using the Nutrient Film Technique. Horticulturae 2022, 8, 1088. [Google Scholar] [CrossRef]
- Renna, M.; D’Imperio, M.; Maggi, S.; Serio, F. Soilless biofortification, bioaccessibility, and bioavailability: Signposts on the path to personalized nutrition. Front. Nutr. 2022, 9, 1–16. [Google Scholar] [CrossRef]
- Buttaro, D.; Renna, M.; Gerardi, C.; Blando, F.; Santamaria, P.; Serio, F. Soilless production of wild rocket as affected by greenhouse coverage with photovoltaic modules. Acta Sci. Pol. Cultus. 2016, 15, 129–142. [Google Scholar]
- Renna, M.; Montesano, F.; Gonnella, M.; Signore, A.; Santamaria, P. BiodiverSO: A Case Study of Integrated Project to Preserve the Biodiversity of Vegetable Crops in Puglia (Southern Italy). Agriculture 2018, 8, 128. [Google Scholar] [CrossRef] [Green Version]








| Code | Legend | Habitat Description |
|---|---|---|
| 16 | Coastal sand dunes and sand beaches | Sand-covered shorelines in general, but in particular, onshore areas of sand created by the action of wind and often colonized and stabilized by communities of coarse maritime grasses. |
| 31 | Heath and scrub | Temperate shrubby areas: Atlantic and alpine heaths, subalpine bush and tall herb communities, deciduous forest recolonisation, and hedgerows. |
| 34 | Dry calcareous grasslands and steppes | Dry thermophilous grasslands of the montane zone on mostly calcareous soil surfaces. |
| 36 | Alpine and subalpine grasslands | Grasslands of the alpine and subalpine levels of the Alps, Pyrenees, Cantabrian range, Jura, Central Massif, and northern Apennines, with very fragmentary outposts in the great Hercynian ranges of middle Europe, and in the Caledonian system of Britain; grasslands of the oro- and cryoro-Mediterranean levels or of the alti-Mediterranean level of the Iberian mountains and of the Apennines. |
| 38 | Mesophile grasslands | Lowland and montane mesophile pastures and hay meadows. |
| 41 | Broad-leaved deciduous forests | Forests and woodlands of native deciduous trees, other than floodplain or mire woods; forests dominated by broad-leaved deciduous trees, but comprising broad-leaved evergreen trees, are included. |
| 42 | Coniferous woodland | Forests and woodlands of native coniferous trees other than floodplain and mire woods; formations dominated by coniferous trees, but comprising broad-leaved evergreen trees, are included. |
| 45 | Broad-leaved evergreen woodland | Mediterranean forests dominated by broad-leaved evergreen trees. Laurel forests of the Atlantic islands. Holly woods. |
| Product Type | Uses | References |
|---|---|---|
| Herbal tea of aerial parts | Aphrodisiac and against hypertension | Senejoux et al. [13] |
| Root decoction | Against dysentery | Işcan et al. [16] |
| Distillate of aerial parts | Liqueur | Heather [26] |
| Whole stems, leaves, and umbels | Borscht (ancient soup) | AA.VV [19] |
| Extract of aerial parts | Against gynaecological and fertility problems | AA. VV. [19] |
| Sedatives of the nervous system | Colombo and Luciano [27] | |
| Antidepressant | Colombo and Luciano [27] | |
| Root infusion | Against impotence and frigidity | Colombo and Luciano [27] |
| Biological Activity | Product Type | References |
|---|---|---|
| Antibacterial | Essential oils | Iişcan et al. [16] |
| Antibacterial | Plant extract | Bahadori et al. [37] |
| Antifungal | Essential oils | Iscan et al. [38] |
| Antifungal | Plant extract | Uysal et al. [17] |
| Bahadori et al. [37] | ||
| Fierascu et al. [39] | ||
| Antioxidant | Plant extract | Benedec et al. [40] |
| Uysal et al. [17] | ||
| Fierascu et al. [39] | ||
| Inactivation of enzymes involved in Alzheimer’s disease | Plant extract | Uysal et al. [17] |
| Bio-herbicide | Plant extract | Fierascu et al. [39] |
| Vasorelaxant | Plant extract | Senejoux et al. [13] |
| Cytotoxic against tumour cells | Essential oils | Maggi et al. [34] |
| Strengths | Weaknesses |
|
|
|
|
|
|
| |
| Opportunities | Threats |
|
|
|
|
| |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matarrese, E.; Renna, M. Prospects of Hogweed (Heracleum sphondylium L.) as a New Horticultural Crop for Food and Non-Food Uses: A Review. Horticulturae 2023, 9, 246. https://doi.org/10.3390/horticulturae9020246
Matarrese E, Renna M. Prospects of Hogweed (Heracleum sphondylium L.) as a New Horticultural Crop for Food and Non-Food Uses: A Review. Horticulturae. 2023; 9(2):246. https://doi.org/10.3390/horticulturae9020246
Chicago/Turabian StyleMatarrese, Eleonora, and Massimiliano Renna. 2023. "Prospects of Hogweed (Heracleum sphondylium L.) as a New Horticultural Crop for Food and Non-Food Uses: A Review" Horticulturae 9, no. 2: 246. https://doi.org/10.3390/horticulturae9020246