Background
The image below shows an early oral squamous cell carcinoma (SCC) on the midlateral border of the tongue, previously diagnosed as a lichenoid reaction to amalgam.
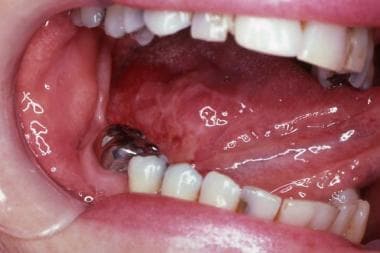 Oral squamous cell carcinoma on the midlateral border of the tongue. Soft to palpation, it serves to illustrate the importance of the differential diagnosis. It was initially misdiagnosed as an allergic reaction (lichenoid lesion) to amalgam
Oral squamous cell carcinoma on the midlateral border of the tongue. Soft to palpation, it serves to illustrate the importance of the differential diagnosis. It was initially misdiagnosed as an allergic reaction (lichenoid lesion) to amalgam
Mouth (oral) cancer is a major neoplasm worldwide and accounts for most head and neck cancers. Theoretically, it should be largely preventable or detectable at an early stage, given that the mouth is easy to access and examine by patients and healthcare professionals, assuming they have good lighting. [1] Approximately 90% of oral cancers are SCC, which is seen typically on the lateral border of the tongue, oropharynx, and floor of the mouth, as a red lesion (erythroplakia), white lesion (leukoplakia), or a mix of the two (erythroleukoplakia) with an ulcer. See the image below.
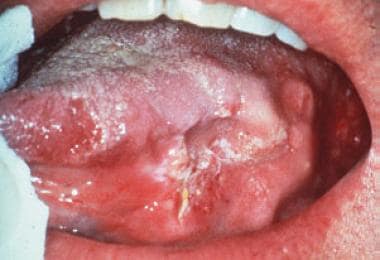 Oral squamous cell carcinoma in the most common intraoral site, lateral tongue, initially reported as a chronic leukoplakia, which had become ulcerated and indurated at the time diagnosis was confirmed.
Oral squamous cell carcinoma in the most common intraoral site, lateral tongue, initially reported as a chronic leukoplakia, which had become ulcerated and indurated at the time diagnosis was confirmed.
Early oral cancer is often asymptomatic, which contributes to delayed diagnosis. Any single ulcerated lesion persisting for more than 2-3 weeks should be regarded with suspicion, and a biopsy should be performed. The mnemonic RULE (red, ulcerated, lump, extending for 3 or more weeks) is an aid to diagnosis. [2, 3]
Oral SCC is particularly common in the developing world, mostly in older males. There is concern about an ongoing increase in younger patients and in women, in particular, without known risk factors, as well as in the oropharynx due to human papillomavirus (HPV) infection. [4] The etiology of oral SCC appears to be multifactorial and strongly related to lifestyle, mostly habits (particularly tobacco alone or in combination with betel, and/or alcohol use), [5, 6, 7] and a poor diet. [8, 9] Other factors such as infective agents may also be implicated, particularly in oropharyngeal cancer (HPV). Immune defects or immunosuppression, defects of carcinogen metabolism, or defects in DNA-repair enzymes underlie some cases of SCC. Sunlight exposure predisposes to lip cancer.
Findings from the history and clinical examination by a trained dentist (oral medicine specialist) are the primary indicators of oral SCC, but the diagnosis must always be confirmed histologically with tissue biopsies, even if the clinical picture is consistent with oral SCC.
Pathophysiology
In oral SCC, modern DNA technology, especially allelic imbalance (loss of heterozygosity) studies, have identified chromosomal changes suggestive of the involvement of tumor suppressor genes (TSGs), particularly in chromosomes 3, 9, 11, and 17. Functional TSGs seem to assist growth control, while their mutation can unbridle these control mechanisms. [10]
The regions most commonly identified thus far have included some on the short arm of chromosome 3, a TSG termed P16 on chromosome 9, and the TSG termed TP53 on chromosome 17, but multiple other genes are being discovered.
As well as damage to TSGs, cancer may also involve damage to other genes involved in growth control, mainly those involved in cell signaling (oncogenes), especially some on chromosome 11 (PRAD1 in particular) and chromosome 17 (Harvey ras [H-ras]). Changes in these and other oncogenes can disrupt cell growth control, ultimately leading to the uncontrolled growth of cancer. H-ras was one of the oncogenes that first caught the attention of molecular biologists interested in cell signaling, cell growth control, and cancer. It and the gene for the epidermal growth factor receptor (EGFR) are involved in cell signaling.
The genetic aberrations involve, in order of decreasing frequency, chromosomes 9, 3, 17, 13, and 11 in particular, and probably other chromosomes, and involve inactivated TSGs, especially P16, and TP53 and overexpressed oncogenes, especially PRAD1. [10]
The molecular changes found in oral SCC from Western countries (eg, United Kingdom, United States, Australia), particularly TP53 mutations, are infrequent in Eastern countries (eg, India, Southeast Asia), where the involvement of ras oncogenes is more common, suggesting genetic differences that might be involved in explaining the susceptibility of certain groups to oral SCC.
Carcinogen-metabolizing enzymes are implicated in some patients. Alcohol dehydrogenase oxidizes ethanol to acetaldehyde, which is cytotoxic and results in the production of free radicals and DNA hydroxylated bases; alcohol dehydrogenase type 3 genotypes appear predisposed to oral SCC. Cytochrome P450 can activate many environmental procarcinogens. Ethanol is also metabolized to some extent by cytochrome P450 IIEI (CYP2E1) to acetaldehyde. Mutations in some TSGs may be related to cytochrome P450 genotypes and predispose to oral SCC. Glutathione S transferase (GST) genotypes may have impaired activity; for example, the null genotype of GSTM1 has a decreased capacity to detoxify tobacco carcinogens. Some GSTM1 and GSTP1 polymorphic genotypes and GSTM1 and GSTT1 null genotypes have been shown to predispose to oral SCC. [11] N-acetyltransferases NAT1 and NAT2 acetylate procarcinogens. N-acetyl transferase NAT1*10 genotypes may be a genetic determinant of oral SCC, at least in some populations.
Tobacco is a potent risk factor for oral cancer. An interaction occurs between redox-active metals in saliva and the low-reactive free radicals in cigarette smoke. The result may be that saliva loses its antioxidant capacity and instead becomes a potent pro-oxidant milieu. [12]
DNA repair genes are clearly involved in the pathogenesis of some rare cancers, such as those that occur in association with xeroderma pigmentosum, but, more recently, evidence of defective DNA repair has also been found to underlie some oral SCCs.
An immune deficiency state may predispose one to a higher risk of developing oral SCC, especially lip cancer.
Etiology
Tobacco and alcohol use are independent risk factors for mouth cancer and tongue cancer. Heavy tobacco smokers have a 20-fold greater risk; heavy alcohol drinkers a 5-fold greater risk; those who do both have a 50-fold greater risk. Betel-quid chewing and oral snuff are important risk factors in people from specific geographic areas (eg, betel chewing in Southeast Asia). Finally, a diet low in fresh vegetables and fruits has also been implicated in causing oral SCC, [8, 13] , and HPVs have been implicated in oropharyngeal cancers. [14] At present, there is not enough evidence to link the use of marijuana (cannabis) with oral cancer, [15] other than the fact that patients who use marijuana tend to have higher rates of infection with HPV. The following factors are associated with the etiology of oral cancers:
-
Cigarette smoking: Compared with persons who do not smoke, the risk of oral cancer in persons who smoke low/medium-tar cigarettes and high-tar cigarettes was 8.5- and 16.4-fold greater, respectively. (Note that cigarettes are classified as low/medium if the tar yield is less than 22 mg and high tar if the tar yield is greater than 22 mg.) See the image below.
-
Alcohol: Growing evidence is associating increased alcohol consumption with the risk of developing oral SCC. [6] Alcoholic beverages may contain carcinogens or procarcinogens, including nitrosamine and urethane contaminants and ethanol. Ethanol is metabolized by alcohol dehydrogenase and, to some extent, by cytochrome P450 to acetaldehyde, which may be carcinogenic. The combined effects of tobacco use and alcohol consumption are found to be multiplicative. Compared with persons who do not drink and do not smoke, the risk of developing oral SCC is increased 80-fold in persons with the highest levels of smoking and alcohol consumption. The most common site of oral SCC among heavy drinkers is the floor of the mouth.
-
Betel and similar habits [16] : The betel quid contains a variety of ingredients, including betel vine leaf, betel (areca) nut, catechu, and, often, slaked lime together with tobacco. Some persons chew the nut only, and others prefer paan, which includes tobacco and sometimes lime and catechu. In 1986, the International Agency for Research on Cancer deemed betel-quid chewing an important risk factor, and the areca (betel) nut habit with or without tobacco use can cause cytogenetic changes in the oral epithelium. Work by Hu et al reported in 2020 suggests a significant increased risk of oral cancer associated with prolonged consumption of betel quid with smoking, and if alcohol is used. [16, 17] Various other chewing habits, usually combinations that contain tobacco, are used in different cultures (eg, Qat, Shammah, Toombak). Tobacco chewing in people from parts of Asia appears to predispose to oral SCC, particularly when it is started early in life and is used frequently and for prolonged periods. [7, 18] Studies from India have confirmed the association between paan tobacco chewing and oral SCC, particularly cancer of the buccal and labial mucosa.
-
Diet: Dietary habits may play a role in the development of oral cancer. A diet rich in fresh fruits and vegetables with limited consumption of meats is recommended for prevention of cancer. Health supplements (vitamins, minerals, and other bioactive compounds) have not been shown to confer the same level of effectiveness in replacing these nutrients. Hence, these health supplements should not be used as substitute for vegetables and fruits in meals. [4, 8, 9, 13]
-
Oral health [19] : A case-control study (ie, every oral cancer case prior to surgery and every control at the time of interview had a structured oral examination) from China found that wearing dentures, per se, is not a risk factor, although the risk was increased in men who wore dentures made from metal. Poor dentition, as reflected by missing teeth, emerged as a strong risk factor independent of other established risk factors. However, poor dentition could lead to poor diet, and hence a higher risk for oral cancer.
-
Socioeconomic status: Behaviors that lead to social instability itself have been linked to an increased risk of oral cancer, but many other explanations may exist (eg, habits, poor oral health and nutrition).
-
Infective agents: Candida albicans and viruses, such as herpes viruses and papillomaviruses, may be implicated in some cases. HPVs are particularly implicated in oropharyngeal cancers. [22] HPV-related tumors tend to be seen in younger patients, in the fauces, and usually have a better prognosis.
-
Others: Associations also are apparent between oral cancer and other various oral conditions (eg, oral submucous fibrosis, oral lichenoid lesions, oral lichen planus, [23] lupus erythematosus, dyskeratosis congenita, Fanconi anemia).
Epidemiology
The oral cavity is one of the 10 most frequent sites of cancer internationally, with three quarters of cases affecting people in the developing world, where, overall, oral cancer is the third most common cancer after stomach and cervical cancer. An estimated 378,500 new cases of intraoral cancer are diagnosed annually worldwide. [4]
Unfortunately, the parts of the world where oral cancer is most common are also those where descriptive information (ie, incidence, mortality, prevalence) is least available. In certain countries, such as Sri Lanka, India, Pakistan, and Bangladesh, oral cancer is the most common cancer. In parts of India, oral cancer can represent more than 50% of all cancers.
The worldwide incidence of oral cancer is estimated to be around 260,000 cases annually, although there is great variation in the incidences across the world. Countries like Taiwan, Hungary, Brazil, France, and parts of South Africa have higher incidences compared with some other countries, such as Japan. In developed countries, oral cancer is less common but is the eighth most common form of cancer overall. For example, in areas of northern France, oral cancer is the most common form of cancer in men. The prevalence of lip cancer appears to be decreasing overall, yet there are regions within the European Union where actinic radiation is responsible for an increase in lip cancer cases (eg, Spain), but the prevalence of intraoral cancer appears to be rising in many countries, especially in younger people. This is especially true in Central and Eastern Europe, especially Hungary, followed by Northern France, which has the highest incidence rate of oropharyngeal cancer. [13] Within the United States, oral cancer represents the eleventh most common cancer in males and the sixteenth most common in females. Approximately 53,260 new cancer cases of the oral cavity and pharynx are estimated by 2020, with about 10,750 patients succumbing to the disease annually (7,760 men and 2,990 women). [24]
Race
The prevalence of tongue cancer is consistently found to be higher (by approximately 50%) in Blacks compared with Whites within the same regions of the United States. [25] Oral and oropharyngeal cancers in Black people have decreased by 1-2% each year since 2007 to 2016. [24] However, oral cancer rates remain higher for Hispanics and Blacks than for White males. However, during the same period, HPV-associated oral and oropharyngeal cancers have increased by about 1% among non-Hispanic Whites. Likewise, the prevalence of oral cancer is generally higher in ethnic minorities in other developed countries. [26]
Sex
Oral cancer affects males more frequently than females, although the ratio is equalizing. For 2020, the estimated number of new cases by sex in the United States is 38,380 for males and 14,880 for females, and estimated deaths for males are 7,760 and for females are 2,990. [24]
Age
Oral cancer is predominantly found in middle-aged and older persons. However, in recent years, an increase in younger patients has been observed.
Prognosis
In general, the prognosis for oral cancer depends on tumor staging and the location of the tumor. At most times, the staging of the tumor is associated with the timing of the diagnosis. The earlier the diagnosis, the lower the tumor stage, and hence, better survival rate (83.7%) is noted compared with a lower survival rate with a late diagnosis, leading to a higher stage III-IV (38.5%). However, other factors also have to be taken into account, such as the location of the tumor, the patient’s general health, age, tobacco and alcohol usage, and the presence of HPV infection.
Based on 2020 data from the United States, [27] the overall 5-year survival rate for oral and pharyngeal cancers at an early stage is 84% (only 29% of all oral cancers are diagnosed at this stage). However, if the cancer has spread to surrounding tissues and lymph nodes, the overall 5-year survival rate is approximately 65%, and 39% if it spreads to distant organs. Lip carcinoma generally has the best 5-year survival rate (88%), owing to early diagnosis, whereas floor-of-the-mouth cancer has the worst rate (54%). [4] Tumor staging, therefore, is the best prognostic factor for intraoral cancers and lip carcinomas, while the status of transcriptionally active HPV is considered the most important prognostic factor for oropharyngeal cancers. In fact, HPV-positive tumors tend to respond better to therapy compared with HPV-negative tumors, and have higher survival rates. [24]
Patient Education
Unfortunately, little has been done in regard to patient education as it concerns oral cancer. Like melanoma, oral cancer can be easily seen, except those in the posterior regions of the tongue, by the patient and the primary care physician. However, this is true only if they know how to identify it. The National Cancer Institute estimates 100,350 new cases of melanoma for 2020, with 6,850 estimated deaths, while for oral cancer, they estimate 53,260 new cases, with a mortality of 10,750. This difference can only be explained by the aggressive campaign sponsored by the America Academy of Dermatology against melanoma, which produced and distributes visual teaching material for patients about the risk of melanoma and clinical presentation. Increased awareness has been instituted by relevant dental societies (eg, American Dental Association, American Academy of Oral Medicine) to educate the public on risk factors and typical clinical presentation of precancerous lesions and oral cancer. Patients should be educated regarding lifestyle changes, including a better diet richer in vegetables and fruits, risk of oral cancer due to oral sex, discontinued smoking, and moderation of alcohol consumption. Furthermore, patients should be encouraged to learn more about their oral condition, how to do a self-oral examination, prevention, treatment options, and complications from therapy.
For patient education resources, see the following:
-
Oral squamous cell carcinoma in the most common intraoral site, lateral tongue, initially reported as a chronic leukoplakia, which had become ulcerated and indurated at the time diagnosis was confirmed.
-
Oral squamous cell carcinoma in the anterior buccal mucosa arising from a chronic candidal-associated leukoplakia. The lesion slowly developed into an indurated lump in a patient with a history of smoking, who thought it was a traumatic lesion.
-
Cancer developing on the gingiva, misdiagnosed as a pyogenic granuloma.
-
Cervical lymph node metastasis from oral cancer.
-
Oral squamous cell carcinoma on the midlateral border of the tongue. Soft to palpation, it serves to illustrate the importance of the differential diagnosis. It was initially misdiagnosed as an allergic reaction (lichenoid lesion) to amalgam
-
This photo illustrates the importance of a thorough oral examination. Oral squamous cell carcinoma on lateral posterior border of tongue. Notice the ulcer, tender at the time of diagnosis, but not indurated, which was missed by the referral doctor. Oral cancer on the posterior border of the tongue is difficult to see unless the tongue is pulled forward.
-
Advanced oral squamous cell carcinoma presenting as a large, ulcerated lump on the left anterior and midlateral border of the tongue.











