Definition
Premalignant squamous lesions of the oral cavity are areas of altered epithelium that are at an increased risk for progression to squamous cell carcinoma (SCC). [1] The most common of these lesions is squamous dysplasia in association with leukoplakia and erythroplakia, which is the primary focus of this article. Not to be ignored, however, is the fact that up to 50% of oral SCCs cases arise from clinically normal mucosa. [2]
Most often, these premalignant conditions manifest clinically as leukoplakia and erythroplakia. Leukoplakia is defined by the World Health Organization as a white lesion of the oral mucosa that cannot be scraped off and cannot be attributed to another definable lesion (see the first image below). [1, 3] Erythroplakia is a red patch on the oral mucosa that cannot be accounted for by any specific disease entity (see the second image below); it exists on a continuum both in appearance and behavior with leukoplakia and mixed erythroleukoplakia (a lesion that is both white and red; see the third image below). [1, 4, 5]
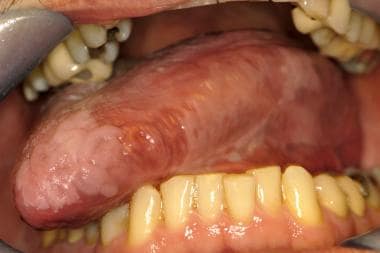 Leukoplakia. Irregular, smooth to thickened leukoplakia involves the dorsal and lateral surfaces of the tongue, which demonstrated no sign of dysplasia in multiple areas of incisional biopsy.
Leukoplakia. Irregular, smooth to thickened leukoplakia involves the dorsal and lateral surfaces of the tongue, which demonstrated no sign of dysplasia in multiple areas of incisional biopsy.
 Erythroplakia. Erythroplakia is characterized by a smooth, velvety clinical presentation with a homogeneous surface, without ulceration. The tissue diagnosis was squamous cell carcinoma in situ.
Erythroplakia. Erythroplakia is characterized by a smooth, velvety clinical presentation with a homogeneous surface, without ulceration. The tissue diagnosis was squamous cell carcinoma in situ.
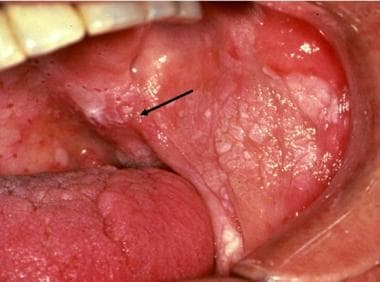 Erythroleukoplakia. Heterogeneous presentation of combined red and white surface alterations are noted, with an intermingling of these changes characteristic of erythroplakia noted at the lateral aspect of the soft palate and buccal mucosal interface (arrow). The tissue diagnosis was squamous cell carcinoma, minimally invasive.
Erythroleukoplakia. Heterogeneous presentation of combined red and white surface alterations are noted, with an intermingling of these changes characteristic of erythroplakia noted at the lateral aspect of the soft palate and buccal mucosal interface (arrow). The tissue diagnosis was squamous cell carcinoma, minimally invasive.
Other less-common conditions that also demonstrate an increased risk for malignant transformation include oral submucous fibrosis (OSF), xeroderma pigmentosum, dyskeratosis congenita, epidermolysis bullosa, iron deficiency, and late-stage syphilis. [6] Proliferative verrucous leukoplakia (PVL) is a specific form of premalignant squamous disease with distinct clinical, histologic, and prognostic features [7, 8] ; although this condition is uncommon, it will be discussed briefly.
Location
Premalignant squamous lesions of the oral cavity most often occur on the buccal mucosa, the mandibular mucosa/sulcus, the palate, the tongue, and the floor of the mouth. [3, 4] Proliferative verrucuous leukoplakia (PVL) is multifocal, with the buccal mucosa the most common initial site of involvement. [6]
Alternative diagnoses
When evaluating suspected premalignant squamous lesions of the oral cavity, clinicians should also consider the following in the differential diagnosis:
-
Radiation atypia
-
Reactive atypia
Etiology
The major risk factors for oral squamous dysplasia—and for squamous cell carcinoma (SCC)—are tobacco smoking and alcohol consumption in Western societies, with other important factors, including the use of areca nut/betel quid in other societies, particularly within India and Southeast Asia, associated with an extremely high oral cancer incidence in these countries (see below). [9] Although such elements are independent risk factors, they are also synergistic with each other. [1, 10, 11]
Other risk factors include immune suppression (eg, head and neck SCC is the third most common malignancy in patients with human immunodeficiency virus [HIV] infection), [12] chronic sun exposure (specifically for cancer of the vermillion border of the lip), and nutritional deficiency. [11]
Far less common, and more controversial, risk factors include oral lichen planus, discoid lupus erythematosus, smokeless chewing tobacco, and poor oral hygiene. [6, 11] Chewing of areca nut (in the form of betel quid), common in some Asian cultures, is a specific risk factor for SCC arising from, or noted in common with, oral submucous fibrosis. [6]
Epidemiology
The prevalence of premalignant oral lesions is approximately 1%-5%. [13] Overall, the rates of oral squamous dysplasia and subsequent squamous cell carcinoma (SCC) are decreasing, closely paralleling the reduction in cigarette smoking. [10] Data from the National Cancer Database show dramatic improvements in 3-year survival of early- and late-stage oral cavity SCC. [14] (This is in contrast to the increasing rates of SCC of the oropharynx, which is predominantly driven by human papillomavirus [HPV] infection [not discussed further in this article].) The improved 2- and 5-year prognosis of early-stage (stages 1 and 2) oral cancer (36%) and late-stage (stages 3 and 4) oral cancer (16%) may be related to negative surgical margins and the performance of neck dissections, whereas improvements in advanced-stage disease has been attributed to the increased use of chemoradiotherapy. [14]
Most cases of leukoplakia and erythroplakia are seen in adults older than 50 years who have risk factors (discussed in Etiology). Males are predominantly affected, with a male-to-female ratio of approximately 3:1; this difference becomes more pronounced with increasing age. [3] However, this ratio appears to have decreased, believed to have resulted from a relative increase of cigarette smoking among women. [15]
Proliferative verrucous leukoplakia (PVL), in contrast, is seen much more often in women (male-to-female ratio, 1:4) in their seventh and eighth decades of life. [6] These patients usually do not have other risk factors for the development of dysplasia and SCC. [4] The etiology of PVL is not known. [6] Of note, this clinical diagnosis is associated with a high rate of malignant transformation, generally considered to be over 70%. [16]
Clinical Features and Imaging
Premalignant squamous conditions most often present clinically as leukoplakia and erythroplakia (see Definition). Leukoplakia is subdivided into homogeneous leukoplakia, nonhomogeneous leukoplakia, and proliferative verrucous leukoplakia (PVL). [3] Homogeneous leukoplakia is the most common type; it usually appears on the buccal mucosa as uniformly white plaques that can be smooth or wrinkled. [3]
Nonhomogeneous leukoplakia is subdivided into speckled and nodular types, both of which can be regarded as erythroleukoplakia (eg, mixture of leukoplakia and erythroplakia). Speckled leukoplakia consists of flecks of white on an erythematous base. Nodular leukoplakia consists of small surface excrescences, often on a red background. [3]
PVL is the least common type of leukoplakia. The lesions initially appear as foci of irregular white patches or plaques with a complex surface but progress slowly and by degrees into multifocal disease with grossly exophytic features (see the image below). [3, 4, 6]
 Proliferative verrucous leukoplakia (PVL). Characteristics of PVL include irregular, thickened, and papillary surface keratotic features with uneven borders. Interdental extension of this process is common in dentate areas.
Proliferative verrucous leukoplakia (PVL). Characteristics of PVL include irregular, thickened, and papillary surface keratotic features with uneven borders. Interdental extension of this process is common in dentate areas.
Erythroplakia is less common than leukoplakia and appears as a fiery red macule or patch with a soft velvety texture. [3, 4] It is associated with a significantly higher risk of dysplasia or carcinoma when compared with typical leukoplakia.
Microscopic Findings
The histologic features of squamous epithelial dysplasia consist of both abnormal architecture and cytologic atypia. Abnormal architectural features seen in dysplasia include loss of cellular organization or stratification, basal cell layer hyperplasia and loss of polarity, dyskeratosis (single-cell keratinization), keratin pearls deep within the epithelium, and drop-shaped rete ridges. [1]
Atypical cytologic features include nuclear enlargement, increased nuclear-to-cytoplasmic ratio, variations in cellular and nuclear size and shape, prominent nucleoli, nuclear hyperchromasia, increased mitotic activity (particularly above the basal layer), and atypical mitotic figures. [1]
Reactive atypia in the oral cavity can have many of the histologic features also seen in squamous dysplasia (eg, nuclear enlargement, increased mitoses, prominent nucleoli). Although these features are usually mild, reactive atypia can occasionally be very difficult to distinguish from true dysplasia.
Despite numerous grading schemes for dysplasia having been advocated, the 2005 World Health Organization (WHO) classification, which divides dysplasia into mild, moderate, severe, and carcinoma in situ, is the one most frequently followed [4] ; however, many pathologists use the terms "severe dysplasia" and "carcinoma in situ" synonymously. [1]
In principle, the grading scheme for squamous dysplasia of the oral cavity is similar to that previously used for the uterine cervix. In mild dysplasia (see the first image below), there is minimal cytologic atypia, and architectural changes are limited to the lower third of the epithelium, whereas in moderate dysplasia (see the second image below), changes are present in the middle third. In severe dysplasia (see the third image below), there is marked atypia, and architectural changes extend to the upper third of the epithelium.
 Mild dysplasia. The squamous epithelium demonstrates atypical architectural features in the form of drop-shaped rete ridges, basal cell hyperplasia, and loss of basal cell polarity. The changes are limited to the lower third of the epithelium.
Mild dysplasia. The squamous epithelium demonstrates atypical architectural features in the form of drop-shaped rete ridges, basal cell hyperplasia, and loss of basal cell polarity. The changes are limited to the lower third of the epithelium.
 Moderate dysplasia. The squamous epithelium shows drop-shaped rete ridges, basal layer hyperplasia and dispolarity, and cytologic atypia. The changes extend about halfway up the thickness of the epithelium.
Moderate dysplasia. The squamous epithelium shows drop-shaped rete ridges, basal layer hyperplasia and dispolarity, and cytologic atypia. The changes extend about halfway up the thickness of the epithelium.
 Severe dysplasia. The atypical features seen here include abnormal maturation, dyskeratotic cells, increased nuclear-to-cytoplasmic ratios, mitotic figures of the basal layer, and cellular pleomorphism. The changes extend into the upper third of the epithelium.
Severe dysplasia. The atypical features seen here include abnormal maturation, dyskeratotic cells, increased nuclear-to-cytoplasmic ratios, mitotic figures of the basal layer, and cellular pleomorphism. The changes extend into the upper third of the epithelium.
Grading of dysplasia in the oral cavity is less rigid, however, than the historical grading of cervical dysplasia. For example, if architectural changes are present in the middle third of the epithelium but accompanied by significant cytologic atypia, the lesion may be classified as severe dysplasia. Similarly, the same amount of architectural distortion with only mild atypia may be downgraded to mild dysplasia. Of note is the concept and practical aspect of interobserver agreement regarding grading of dysplasia where a study by Speight et al found a low level of interobserver and intraobserver agreement with regard to the histopathology of oral epithelial dysplasia and the absence of a so-called gold standard of reliable diagnostic methodology. [17]
Technically, carcinoma in situ—defined as full-thickness architectural changes with marked cytologic atypia—does not exist in keratinized squamous mucosa. Accordingly, many pathologists use the term synonymously with severe dysplasia, as previously noted.
Of particular mention, anecdotal reports emphasize that carcinoma may develop in the absence of demonstrable dysplasia; thus, clinicians are urged to maintain active surveillance of clinically suspicious lesions. [18]
Proliferative verrucous leukoplakia (PVL) is characterized histologically across a wide spectrum of alterations, including verrucous hyperplasia—a corrugated squamous epithelial surface with “church-spire” hyperkeratosis in well-developed lesions (see the image below) [4] —to cases in which variable levels of dysplasia are present, to frank carcinoma of verrucous or squamous type.
 Proliferative verrucous leukoplakia (PVL). Depicted is squamous epithelium displaying an accentuated, scalloped, verrucoid, keratinized hyperplasia. Although this is a nonspecific finding, in the appropriate clinical context it is consistent with PVL.
Proliferative verrucous leukoplakia (PVL). Depicted is squamous epithelium displaying an accentuated, scalloped, verrucoid, keratinized hyperplasia. Although this is a nonspecific finding, in the appropriate clinical context it is consistent with PVL.
Early PVL lesions are often very subtle, necessitating a high index of suspicion. The lesions become progressively more florid and atypical before developing into verrucous carcinoma or frank invasive squamous cell carcinoma (SCC). It must be emphasized, however, that PVL is a clinical entity and that clinicopathologic correlation is therefore essential in establishing the diagnosis.
Immunohistochemistry
The role of immunohistochemistry in the diagnosis of oral premalignant squamous lesions is currently very limited. Ki-67 (MIB-1), a marker of proliferation, may be of some value when histologic findings are ambiguous; increased Ki-67 expression, particularly in the upper two thirds of the epithelium, may tend to favor a diagnosis of dysplasia. [19, 20, 21, 22]
Some studies have shown an increased expression of some cytokeratins (CKs), especially CK 8/18 and CK19, in oral dysplasia. [23, 24, 25] Other research has revealed an increased expression of p53 and p16 in dysplastic lesions. [20, 21, 22, 26, 27]
None of these markers is entirely sensitive or specific for dysplasia, however, and their use is typically limited to gathering additional information in difficult, borderline cases.
Molecular/Genetics
The transition from normal epithelium to premalignant change to squamous cell carcinoma (SCC) is thought to result from an accumulation of genetic changes. [28] Observed alterations in the progression of oral SCC include overexpression of EGFR, cyclin D1, matrix metalloproteinase 9, and p53; aneuploidy; upregulation of telomerase; and losses of heterozygosity at chromosome loci 3p and 9p. [28, 29, 30]
Although the use of these markers is potentially very attractive (particularly in the face of high interobserver variability in histologic interpretation), none of these molecular alterations has yet been shown to be uniformly predictable in routine practice, either individually or in combination. [28] There has been research regarding quantification of genetic abnormalities at the level of copy number variation at several chromosomal loci, where such accrual of copy variations has been associated with progressive levels of dysplasia. [31]
Prognosis and Predictive Factors
The term "leukoplakia" is often used as if it were synonymous with “premalignant condition,” but it is important to remember that only a distinct minority of leukoplakias harbor dysplasia or carcinoma. [3, 32] Homogeneous leukoplakia transforms to malignancy in only about 6% of cases. [33] Nonhomogeneous leukoplakia and erythroplakia, although less common, have a much higher rate of dysplasia, with at least 85% of cases showing severe dysplasia or frank squamous cell carcinoma (SCC). [3, 34]
Note that lesions of the floor of the mouth and the lateral and ventral tongue are more likely to represent dysplasia and that premalignant lesions of these sites are at a higher risk for malignant transformation than similar lesions at other sites in the oral cavity. [4, 13]
The ultimate goal of accurately recognizing and grading squamous dysplasia of the oral cavity is to communicate to the clinician the expected biologic behavior of the lesion in order to guide management. However, the criteria for diagnosing the various grades of dysplasia are subjective; it is well known that current grading systems for oral dysplasia are imperfect, with many studies showing interobserver and intraobserver agreement that is fair, at best. [35]
Follow-up studies examining the progression of dysplasia to carcinoma have found that patients with any dysplasia progress to cancer in 5%-36% of cases. [33, 36, 37, 38, 39, 40] Although it would seem intuitively likely that the risk of malignant transformation would increase in tandem with increasing severity of dysplasia (as has been shown to be the case in the larynx, for example), such a progression has not been well established in the oral cavity.
Some evidence already exists to the contrary—namely, that severe dysplasia or carcinoma in situ is not a prerequisite for the development of invasive SCC in the oral cavity—in that SCC may develop from cases of even mild dysplasia (so-called drop-off or drop-down carcinomas). From a clinical perspective concerning the malignant transformation of oral leukoplakia, several factors may play a role in this process including advanced age, female sex, surface area of leukoplakia exceeding 200 mm2, nonhomogeneous qualities (so-called erythroleukoplakia), and higher grade of dysplasia. [41]
Proliferative verrucous leukoplakia (PVL), in contrast, is a slowly progressive, multifocal disease that eventually progresses to SCC in almost all cases. [3, 4, 6] The SCC may be of either the conventional or the verrucous subtype. [4, 6]
-
Leukoplakia. Irregular, smooth to thickened leukoplakia involves the dorsal and lateral surfaces of the tongue, which demonstrated no sign of dysplasia in multiple areas of incisional biopsy.
-
Erythroleukoplakia. Heterogeneous presentation of combined red and white surface alterations are noted, with an intermingling of these changes characteristic of erythroplakia noted at the lateral aspect of the soft palate and buccal mucosal interface (arrow). The tissue diagnosis was squamous cell carcinoma, minimally invasive.
-
Erythroplakia. Erythroplakia is characterized by a smooth, velvety clinical presentation with a homogeneous surface, without ulceration. The tissue diagnosis was squamous cell carcinoma in situ.
-
Proliferative verrucous leukoplakia (PVL). Characteristics of PVL include irregular, thickened, and papillary surface keratotic features with uneven borders. Interdental extension of this process is common in dentate areas.
-
Mild dysplasia. The squamous epithelium demonstrates atypical architectural features in the form of drop-shaped rete ridges, basal cell hyperplasia, and loss of basal cell polarity. The changes are limited to the lower third of the epithelium.
-
Moderate dysplasia. The squamous epithelium shows drop-shaped rete ridges, basal layer hyperplasia and dispolarity, and cytologic atypia. The changes extend about halfway up the thickness of the epithelium.
-
Severe dysplasia. The atypical features seen here include abnormal maturation, dyskeratotic cells, increased nuclear-to-cytoplasmic ratios, mitotic figures of the basal layer, and cellular pleomorphism. The changes extend into the upper third of the epithelium.
-
Proliferative verrucous leukoplakia (PVL). Depicted is squamous epithelium displaying an accentuated, scalloped, verrucoid, keratinized hyperplasia. Although this is a nonspecific finding, in the appropriate clinical context it is consistent with PVL.










