Ectopic pregnancy (EP) represents an important cause of acute pelvic pain in women of reproductive age. The incidence of EP has increased more than threefold since the 1970s, likely the result of a combination of factors, including an increase in the number of patients with risk factors for EP but also earlier detection and improved technology. Conversely the mortality rate has decreased during this time, also likely, in large part, to the result of earlier detection with transvaginal ultrasound (TVUS). EP remains the leading cause of maternal death during the first trimester, however, with a mortality rate 4 times higher than that of normal childbirth. Historically women with EPs presented with life-threatening hemorrhage and were rapidly taken to the operating room for diagnosis and treatment. Currently the diagnosis of EP is made primarily with TVUS, with as many as 90% of clinically relevant EPs detected on initial sonographic examination. In addition, most women with EP are stable at the time of presentation compared with only one quarter in 1978, and more than two thirds of EPs are diagnosed before rupture. Furthermore, routine emergency surgery is no longer required for many patients, and patients are successfully treated with medical therapy, minimally invasive procedures, and even expectant management.
Disease
Definition
EP is the implantation of an embryo outside of the endometrial cavity of the uterus. A woman with heterotopic pregnancies has concurrent intrauterine and extrauterine pregnancies. Another important related term is pregnancy of unknown location (PUL), which is used to describe the clinical scenario of a hemodynamically stable patient with a positive pregnancy test and a TVUS that demonstrates no evidence of either an intrauterine or extrauterine pregnancy.
Persistent EP refers to the presence of a residual EP after therapy, either surgical or nonsurgical. An increasing β-human chorionic gonadotropin (hCG) titer in a treated patient after an initial decrease is indicative of a persistent EP.
Prevalence and Epidemiology
There has been a significant increase in the incidence of EP during the past 40 years. Currently approximately 2% of all pregnancies are ectopic in location, whereas the incidence was only 0.5% in 1970. Conversely the overall mortality rate has decreased by approximately 90% between 1979 and 1992, likely the result of improved methods of detection and treatment. The case fatality rate has shown a corresponding decrease, from 69% in 1876 to 0.35% in 1970 to 0.05% in 1986. EP remains the leading cause of maternal death during the first trimester, however, with a mortality rate between 9% and 14%. The mortality rate is highest for advanced abdominal EPs, for which it approaches 20%. The death rate for minorities with EP is twice that of white women, and 15- to 19-year-old female patients with EPs have the highest fatality rate.
Any abnormality that alters the structure or function of the fallopian tubes is a major risk factor for the development of an EP, including previous infections (usually pelvic inflammatory disease [PID]), previous surgery (especially tubal ligation), or trauma (e.g., previous EP).The greatest risk factor in fact is a history of previous EP, which increases the risk for EP by a factor of 10. A history of PID increases a woman’s risk for EP sevenfold. Other risk factors include pregnancy achieved through assisted fertility techniques, congenital müllerian duct abnormalities, advanced maternal age, history of previous cesarean section, endometriosis, and diethylstilbestrol exposure in utero. The use of contraception reduces a woman’s risk for EP by reducing her overall chance of becoming pregnant. A woman who becomes pregnant with an intrauterine device (IUD) in place, however, has a significantly increased risk for that pregnancy being ectopic. Another important factor contributing to the substantial increase in the number of EPs is the increased use of assisted fertility techniques. Reportedly 2.2% to 5.6% of pregnancies achieved through assisted reproductive technologies are ectopic, and as many as 3% are heterotopic.
Etiology and Pathophysiology
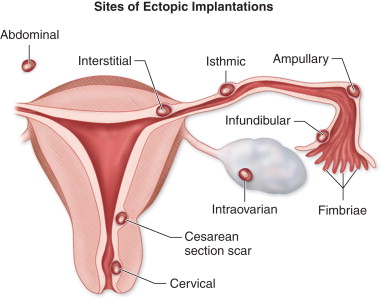
Fertilization of an ovum normally occurs in the fallopian tube. Then the fertilized egg travels down the fallopian tube to implant within the endometrial cavity approximately 6 days after fertilization. Any delay or obstruction to transit within the fallopian tube can lead to ectopic implantation. The vast majority, 95% to 99%, of EPs are located within the fallopian tube. Approximately 70% to 80% of EPs implant in the ampulla, 10% to 15% in the isthmus, 5% to 11% within the fimbriated portion of the tube, and 2% to 4% in the interstitial or intramural portion. Bleeding into the lumen or wall of the fallopian tube frequently results in a masslike hematoma or hematosalpinx. Much less commonly, EPs may implant within the cervix, a cesarean section scar, the ovary, or the abdomen. The most feared and ominous complication of EP is rupture and hemorrhage, which can be life threatening and is more severe the older or the larger the EP at the time of rupture.
Manifestations of Disease
Clinical Presentation
Patients with EP typically present early in the first trimester. The classic clinical triad of pain, vaginal bleeding, and a palpable adnexal mass is observed in only 45% of patients. Additionally, these “classic symptoms” are nonspecific, with only a 14% positive predictive value for EP. Thus it is estimated that between 70% and 90% of pregnant women who present to the emergency department with such symptoms and are clinically suspected of having an EP will eventually be diagnosed with an intrauterine pregnancy (IUP). Although some patients may be asymptomatic, the most common presenting symptom of patients with EPs is pelvic pain. The severity of the pain, however, does not correlate with either the size or rupture of the EP, although some women report a decrease in pain after rupture. Vaginal bleeding is the second most common presenting symptom, occurring in 56% to 79% of patients. Lastly, less than 50% of women will have adnexal tenderness on examination.
Imaging Indications and Algorithm
Any pregnant patient presenting in the first trimester with abdominal pain and/or vaginal bleeding warrants evaluation with TVUS. TVUS is the gold standard for the diagnosis of EP and other causes of pelvic pain and vaginal bleeding, such as miscarriage, subchorionic hemorrhage, hemorrhagic cysts, and ovarian torsion. The sensitivity of initial TVUS for the diagnosis of EP is between 74% and 84%. Recent literature reports that serial TVUS examinations have a sensitivity of 84% to 99%, a specificity of 84% to 99.9%, a positive predictive value of 93.5%, and a negative predictive value of 99.8% for diagnosing EP. It is important to remember, however, that between 5% and 27% of patients with EPs will have a normal initial ultrasound (US) examination, particularly if the patient presents early (3 to 5 weeks of gestational age).
The serum β-hCG level plays an important role in assessing the symptomatic pregnant woman in the first trimester. The current measurement reference for serum β-hCG level is the third international standard, also known as the International Reference Preparation (IRP). In women with EP, the serum β-hCG is usually lower for a given gestational age and increases more slowly than with a normal IUP. In a normal IUP, the β-hCG doubles approximately every 2 days, with a minimum increase of 53% to 66%. If the increase in β-hCG level is less than 53% in 48 hours, the pregnancy is likely abnormal—either an EP or a failed IUP. Approximately 85% of normal IUPs will have an increase of at least 66% over 48 hours. Up to 21% of women with EPs, however, will also have an increase of at least 66% in 48 hours, indicating that serial β-hCG levels may not discriminate normal IUPs from EPs in all patients.
Approximately 8% to 31% of symptomatic women in the first trimester will be described as having a PUL after TVUS. The precise percentage depends both on the skill of the sonographer and the gestational age at presentation because earlier pregnancies are more difficult to visualize whether intrauterine or ectopic in location. In the remaining 69% to 92% of symptomatic pregnant women, the diagnosis of normal or abnormal IUP or EP should be recognizable on the initial US examination in conjunction with laboratory values. One third of PULs represent early developing IUPs, whereas two thirds ultimately turn out to be either failed IUPs or EPs. Approximately 9% to 14% of PULs are EPs, whereas 44% to 69% will fail and regress spontaneously. Those that resolve include both spontaneous abortions (SABs) and failed EPs. It is important to note, however, up to 43% of PULs develop into EPs when the scanning is performed by inexperienced clinicians.
Imaging Technique and Findings
Radiography
No indications exist at present for radiography in the diagnosis of EPs.
Ultrasound
General Overview
Accurate diagnosis of an EP requires the use of TVUS. TVUS should be performed with an empty bladder using the highest possible frequency transducer, usually between 5 and 10 MHz. Most EPs are found between the cornua of the uterus and the ovary. EPs may be found anywhere in the adnexa, however, and are contralateral to the corpus luteum in approximately one third of cases. If an adnexal mass is visualized, a bimanual scanning approach, with one hand manipulating the US probe and the other hand applying pressure to the lower abdominal wall, can help differentiate an exophytic ovarian mass or corpus luteum (the most common mimicker of an EP) from an extraovarian mass, more likely to represent an EP (described in more detail below). Because the cul-de-sac is the most dependent portion of the female pelvis, it is also a common place for an EP to be found. Targeted probing in the area of maximal tenderness will increase the chance of visualizing the EP. Limited transabdominal imaging is useful to evaluate the adnexa or the area of the patient’s pain, if incompletely visualized on TVUS (e.g., anteriorly or superiorly). Color Doppler interrogation of the adnexa may increase the conspicuity of the trophoblastic tissue in an adnexal mass or tubal ring (discussed in the following section).
Additionally the cul-de-sac, Morrison’s pouch, and perirenal areas should generally be assessed with transabdominal imaging to look for free fluid containing low-level internal echoes or clot, suggesting hemorrhage. When evaluating free fluid, the gain setting should be optimized such that real echoes are not erased (gain too low) but artifactual echoes are not created (gain too high). To set the gain appropriately, one should increase the gain while imaging urine in the bladder until artifactual echoes are observed. The gain is then decreased until internal echoes are no longer apparent within the bladder lumen.
A major role of US in evaluating a patient with clinical suspicion for EP is to establish the presence of an IUP because 70% to 90% of patients presenting with pelvic pain and/or vaginal bleeding in the first trimester will ultimately be diagnosed as having an IUP. The documentation of an IUP virtually excludes the possibility of an EP in the general population because the incidence of heterotopic pregnancies in patients without risk factors for EP is extremely low, between 1 in 7000 and 30,000 pregnancies. A thorough discussion of the sonographic and quantitative serum β-hCG criteria for diagnosis of an early IUP is presented in Chapter 21 . For a singleton gestation, the most commonly used threshold values for visualization of an intrauterine gestation sac is a serum β-hCG level between 1800 and 2000 mIU/mL (IRP), although published thresholds vary between 1500 and 2500 mIU/mL (IRP). Approximately 50% of EPs, however, present with β-hCG levels less than 1500 mIU/mL, in other words, with levels below the threshold where one would expect to visualize an IUP. Many of these EPs are visible on initial TVUS examination because β-hCG levels are lower for an EP than for an IUP at a given gestational age.
If an IUP is not found in a symptomatic pregnant woman with a serum β-hCG above the threshold level for visualization of an IUP, the patient should be strongly considered to have either an EP or miscarriage unless the patient is at risk for multiple gestations, in which case an early gestation remains possible. (The β-hCG is higher for a given gestational age in the case of multiple gestations, and therefore the threshold values established for a singleton gestation do not apply.) If the patient does not have a history of vaginal bleeding to suggest miscarriage, EP becomes more likely.
Tubal Pregnancy
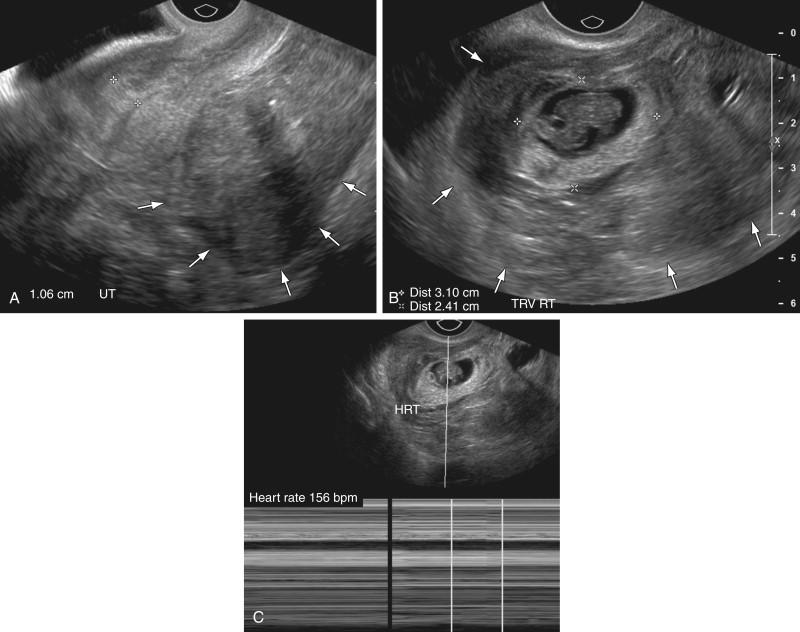
If there is no evidence of an IUP on TVUS examination, attention turns to the adnexa. Visualization of an extrauterine gestational sac containing an embryo ( Figure 22-1 ) or yolk sac (see Figure 22-2 ), with or without the presence of cardiac activity ( Figure 22-1 , C ), is 100% specific for the diagnosis of EP. This finding, however, is present in only 8% to 32% of patients.
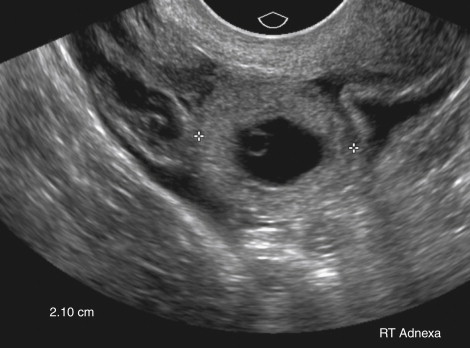
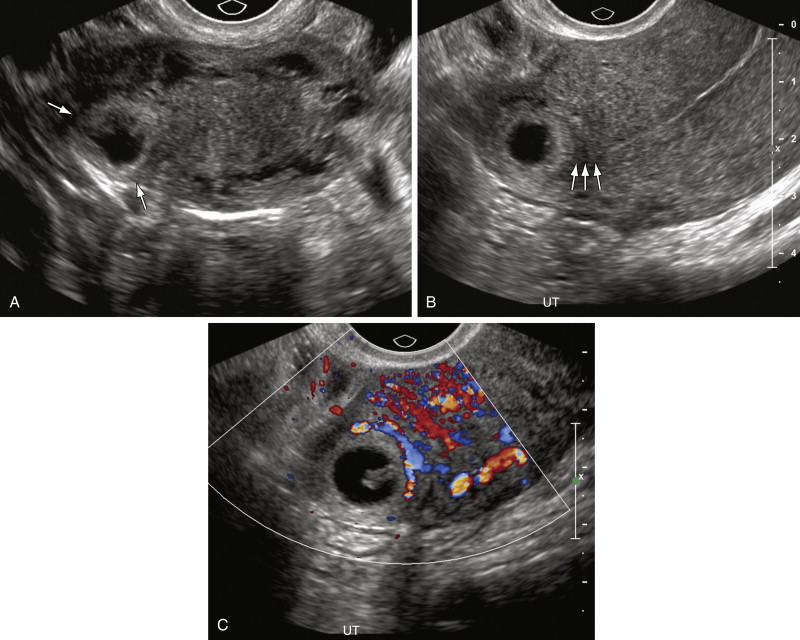
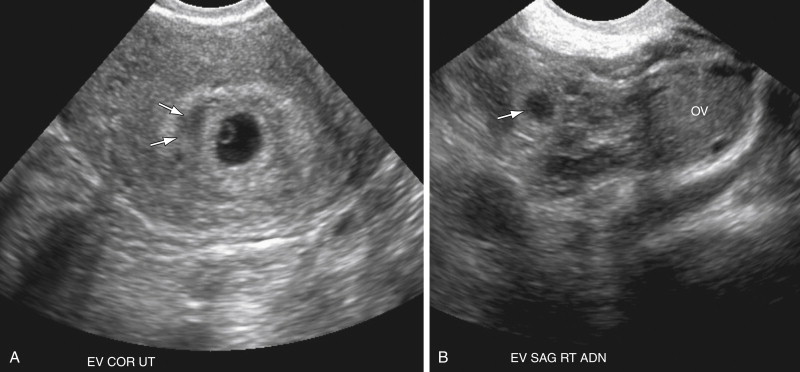
The next most specific finding is an adnexal echogenic ringlike structure surrounding a central fluid collection, the so-called tubal ring ( Figure 22-3 ). This is the second most common finding of EP on US examination and is reportedly observed in 40% to 68% of cases. A tubal ring is typically more echogenic than the central ovarian stroma and similar in echogenicity to the endometrium. The echogenicity of the wall of the tubal ring is variable, however, and there is considerable overlap with the echogenicity and appearance of a corpus luteum (see discussion in the following paragraph). If the tubal ring is clearly distinct from the ovary (see Figure 22-3 , A ) or can be separated from the ovary by the bimanual scanning technique, the specificity of this finding is increased. Acute angles between an adjacent round echogenic ringlike structure and the ovary also suggest that the mass is separate from the ovary (see Figure 22-3 , B ). In some cases, however, an EP may appear to be attached to the ovary as a result of adhesions or surrounding hematoma, and therefore can mimic the US appearance of an exophytic corpus luteum. If a tubal ring is identified, a thorough effort must be made to search for a yolk sac because visualization of a yolk sac within the tubal ring will increase the specificity of the TVUS examination for EP to 100%. Thus the gray scale imaging parameters must be optimized, including magnifying the image, placing the focal zone at or just below the area of interest, increasing the gain, imaging from multiple angles, applying pressure to the anterior abdominal wall to move the structure closer to the transducer and eliminate artifact from overlying bowel gas, and using harmonic imaging and spatial compounding.
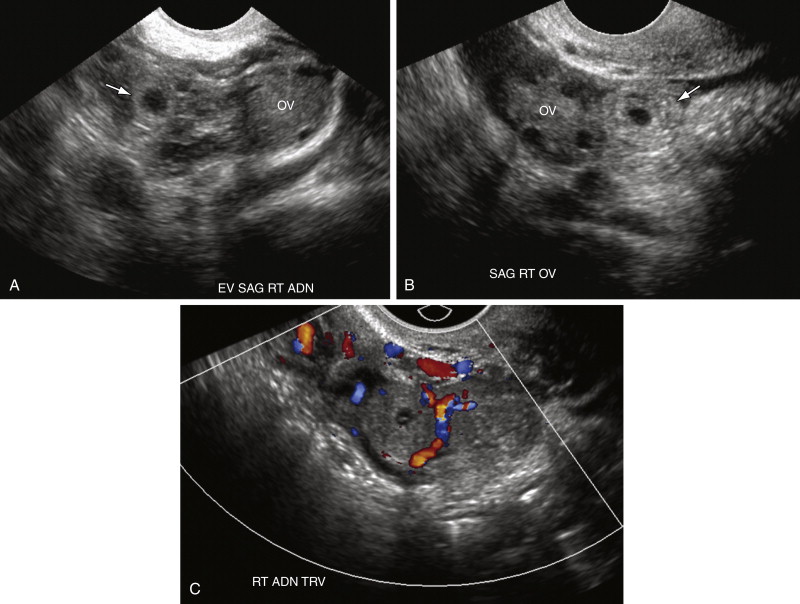
Although the tubal ring was initially described as being vascular, the so-called “ring of fire,” that is, completely circumferential vascularity, is seen in the minority of tubal rings. Many EPs in fact demonstrate merely focal (see Figure 22-3 , C ) or even minimal to completely absent vascularity on color Doppler US examination. Circumferential marked vascularity, however, is common in the wall of a corpus luteum, which can therefore mimic the appearance of an EP.
Lastly the most common but least specific sonographic finding of EP is the presence of solid extraovarian adnexal mass. Such adnexal masses usually represent hematoma in or around the EP and are reportedly observed in 69% to 89% of cases ( Figure 22-4 ). Adnexal hematomas from EPs may vary in echogenicity from hypoechoic to echogenic and may be either homogeneous or heterogeneous, depending on the time since hemorrhage occurred. If the hematoma is contained within the fallopian tube, it may appear tubular in configuration (see Figure 22-4 , B ). Vascularity is variable, with some hematomas being completely avascular on Doppler interrogation. The presence of trophoblastic flow—arterial waveforms characterized by relatively high end diastolic and peak systolic blood flow—however, increases the specificity of the finding. Color Doppler interrogation may sometimes depict the vascular ring/wall of an EP within an adnexal hematoma.
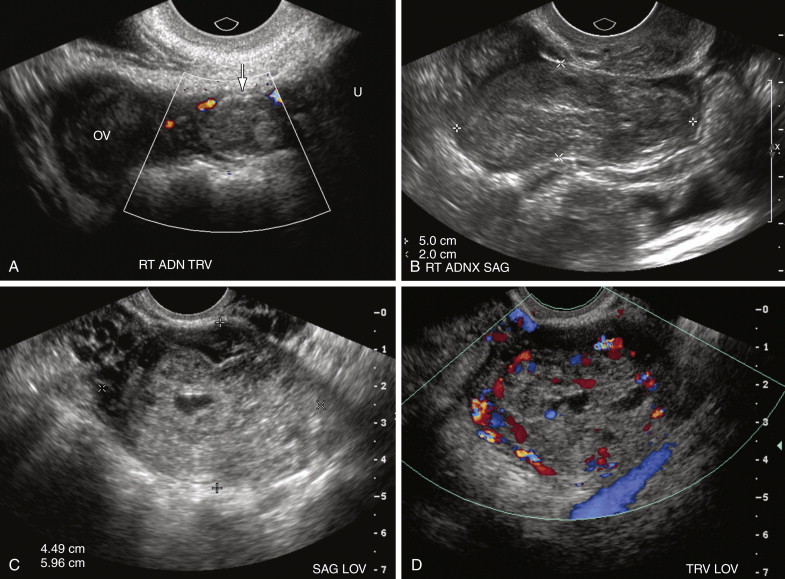
Overall the presence of any of the extraovarian masses described above in a pregnant patient with an empty uterus and serum β-hCG level above the discriminatory level for visualization of an IUP has been reported to be 84% sensitive and 99% specific for the diagnosis of EP, with positive and negative predictive values of 96% and 95%, respectively.
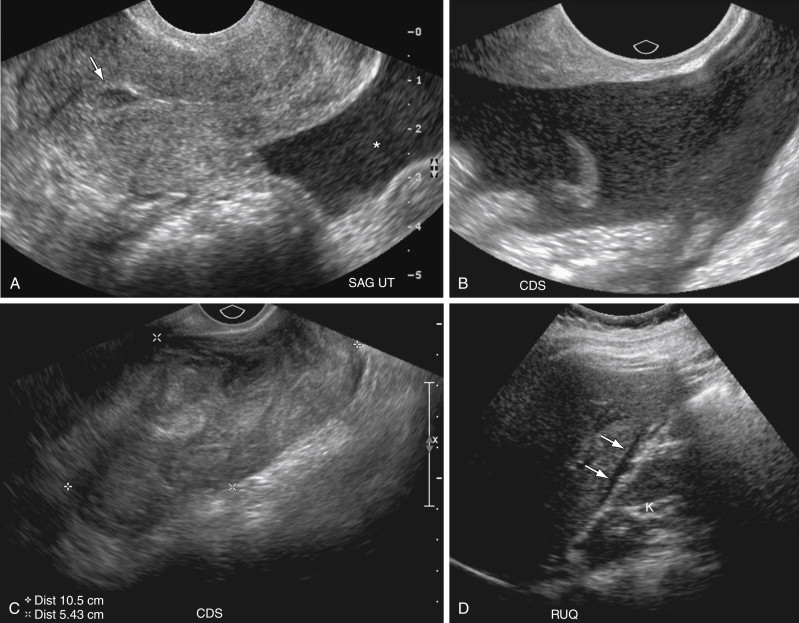
Between 15% and 35% of patients with EP, however, may not have an identifiable adnexal mass on TVUS, and therefore the secondary finding of hemorrhagic free fluid ( Figure 22-5 ) is often crucial for diagnosis. Low-level echoes (see Figure 22-5 , A and B ) or clumped echogenic material (see Figure 22-5 , C ) within the free fluid is suggestive of hemorrhage and clot, respectively, although acute intraperitoneal hemorrhage can be completely anechoic on US examination. Echogenic free fluid in the pelvis has been reported in 63% to 70% of patients with EP and has been observed to be the only positive finding in as many as 15% of patients. The finding of echogenic free fluid is nonspecific, however, with free pelvic fluid seen in 25% to 31% of patients with normal IUPs as a result of retrograde bleeding or rupture of the corpus luteal cyst (see Figure 22-5 , B ). Furthermore, echoes within pelvic fluid may be due to infection or debris. Additionally the amount of free fluid is important for two reasons. First, studies have shown that a moderate to large amount of echogenic free fluid in a pregnant patient has a higher positive predictive value of 86% to 93% for EP. Second, a large amount of hemoperitoneum may herald potential hemodynamic instability and affect patient management. Therefore when free fluid is detected in the pelvis, the upper abdomen, including Morrison’s pouch, should be evaluated for free fluid. The presence of fluid in Morrison’s pouch implies a relatively large amount of bleeding (see Figures 22-5 , D , and 22-14, C , later in the chapter). Although a large amount of hemorrhagic free fluid raises concern for rupture of an EP, there are no reliable sonographic signs of rupture, and significant hemorrhagic free fluid can be seen with nonruptured EPs and even ruptured ovarian cysts (see Figure 22-21 later in the chapter).
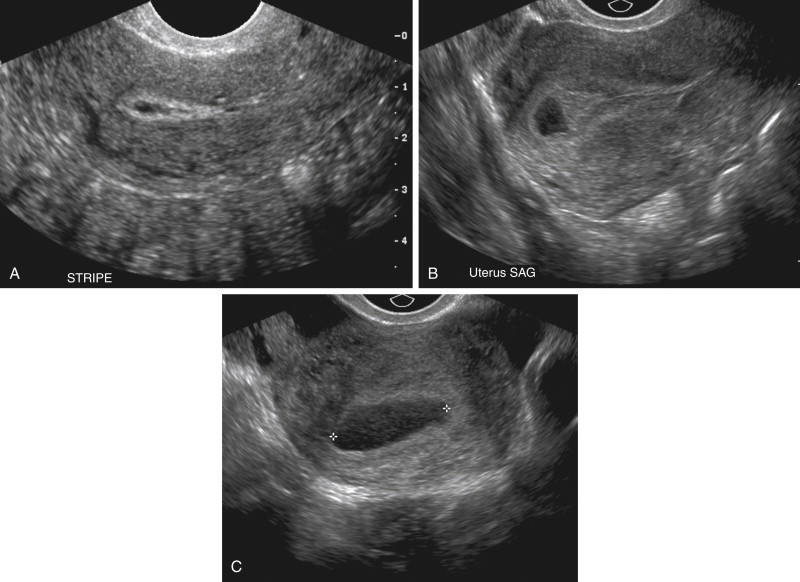
Some patients with an EP will have fluid located centrally within the endometrial canal surrounded by a prominent echogenic decidual reaction that has been described as a pseudogestational sac (see Figures 22-5, A , and 22-6 ). The fluid is caused by bleeding from the decidualized endometrium and is observed in 5% to 20% of patients with EP. Occasionally debris, clot, or sloughed endometrial tissue may mimic the appearance of a yolk sac or embryo within a pseudogestational sac. Careful evaluation should demonstrate that the fluid of a pseudogestational sac is located centrally within the endometrial canal, as opposed to the fluid within a normal intrauterine gestational sac that is located eccentrically within the endometrium, separate from the endometrial canal. Additionally the echogenic rim that is typical of a normal intrauterine gestational sac is usually absent with a pseudogestational sac. Fluid within the endometrial canal, however, is a nonspecific finding with a high false-positive rate for EP and cannot be used alone to diagnose EP. Decidual cysts, which are tiny, thin-walled cysts at the junction of the endometrium and myometrium, are no longer considered a specific finding for EP.
There are no pathognomonic color or spectral Doppler findings associated with EP. Color Doppler imaging, however, can be a useful adjunct in evaluating the adnexa. Identification of vascularity on color flow imaging within an adnexal hematoma will exclude the possibility that the hematoma represents merely bland hemorrhage from a ruptured corpus luteal cyst and suggests the presence of underlying trophoblastic tissue (see Figure 22-4 , C and D ). Color Doppler interrogation may sometimes depict the vascular ring/wall of an EP within an adnexal hematoma that appears completely amorphous on gray scale imaging. Additionally, color Doppler imaging has rarely been reported to document the presence of a focal area of vascularity at the presumed site of an EP in the absence of a clearly visualized mass on gray scale examination. In terms of management, lack of flow by color Doppler US in a mass likely representing an EP may indicate that the pregnancy has involuted and could therefore be managed expectantly or conservatively. More research, however, is needed in this area, and currently Doppler characteristics of an EP are not considered to be major criteria for determining patient management.
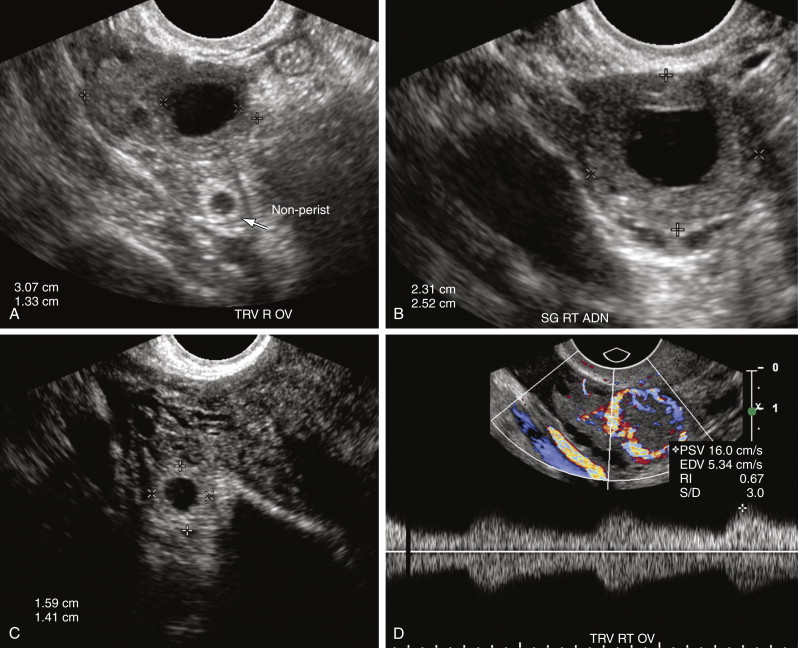
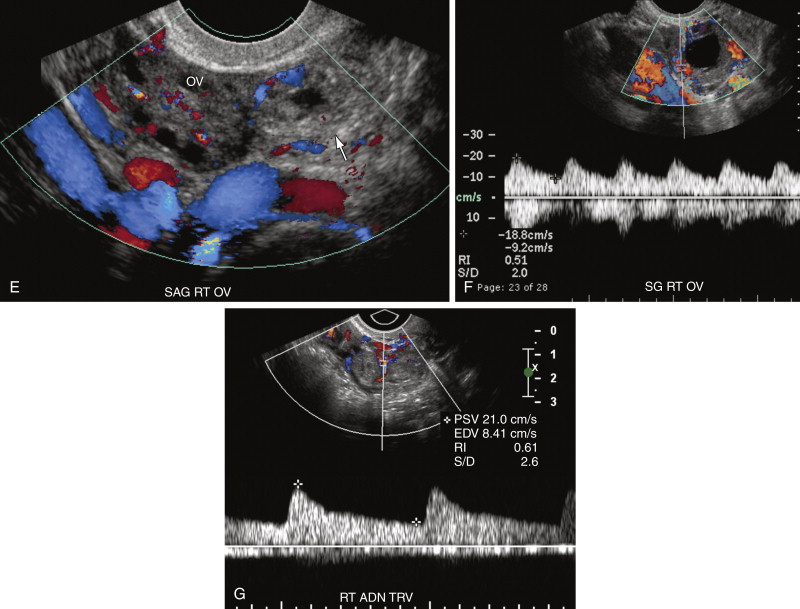
One of the most common pitfalls in diagnosing an EP is differentiating an exophytic corpus luteum from an adnexal mass representing an EP. Because fewer than 3% of EPs are intraovarian, if an adnexal mass is located within the ovary, it is most likely the corpus luteum. The presence of ovarian parenchyma partially surrounding the mass, the so-called “claw sign” ( Figure 22-7 , A ), suggests that the mass is ovarian in origin. In general the wall of a corpus luteum tends to be more hypoechoic, thicker, homogeneous, and symmetric compared with an EP (see Figure 22-7 , A and B ). The corpus luteum often has a crenated or starlike central configuration as it involutes. The wall of an EP is usually more echogenic than the ovarian stroma and similar in echogenicity to the endometrium (see Figure 22-7 , A and C ). However, there is overlap in appearance. Hemorrhage into a corpus luteum may sometimes mimic the presence of a yolk sac or embryo. Dynamic scanning with manual pressure applied to the lower abdominal wall over the ovary while scanning transvaginally may help differentiate an intraovarian from an extraovarian mass. If the mass moves with the ovary, it is likely intraovarian or possibly adherent secondary to adhesions or hematoma. If the mass and the ovary move separately, however, the mass is more likely to be extraovarian and an EP. Neither the color Doppler appearance nor the spectral Doppler waveform is useful in differentiating the corpus luteum from an EP because there is considerable overlap in the Doppler appearance of these structures (see Figure 22-7 , D through G ). Both may have an extremely vascular wall, the “ring of fire,” with a low resistance arterial waveform pattern characterized by relatively high peak systolic velocity (PSV), high end diastolic velocity (EDV), and low resistive index (<0.6). Typically color Doppler interrogation shows less completely circumferential flow in the tubal ring of an EP than in the wall of a corpus luteum. Some EPs have no detectable vascularity on Doppler interrogation.
Other imaging pitfalls that may be encountered in sonographic evaluation of EPs include examinations limited by obesity or early gestational age where the pregnancy is too small to be seen whether intrauterine or extrauterine in location, limited experience on the part of the sonographer, patients with an enlarged uterus especially if distorted by fibroids and displacing the ovaries outside of the true pelvis, and patients with coexistent adnexal pathology.
The presence of an IUP virtually excludes the possibility of an EP pregnancy in the general population because heterotopic pregnancies are extremely rare with an incidence between 0.003% and 0.05% in naturally occurring pregnancies in women without risk factors for EP. Heterotopic pregnancies are much more common in women with risk factors for EP, however, especially women undergoing assisted reproduction techniques, with an estimated incidence between 1% and 3%. Therefore in this subgroup of patients, even if an IUP is confidently identified, a thorough search must be undertaken to exclude coexistent EP ( Figure 22-8 ).
Although most EPs occur within the ampullary portion of the fallopian tube, EPs may also implant in the interstitial portion of the fallopian tube, myometrial scars, the cervix, and, less commonly, within the ovary or abdominal cavity. Because the location of the EP has important implications for patient management and prognosis, it is extremely important to accurately recognize EPs in these unusual locations ( Figure 22-9 ).
Interstitial Pregnancy
A pregnancy implanted in the intramural or interstitial portion of the fallopian tube is termed an interstitial EP . The term cornual pregnancy has sometimes been used synonymously with interstitial pregnancy, but we consider cornual pregnancy to be different, as we discussed later in this section. The presence of surrounding myometrium affords better blood supply and allows an interstitial EP to grow larger before becoming symptomatic. Therefore interstitial EPs frequently present with a high initial serum β-hCG level and come to clinical attention later, at approximately 9 to 12 weeks of gestational age. Interstitial pregnancies are associated with more severe hemorrhage and higher morbidity and mortality rates because of their enhanced blood supply and larger size at the time of rupture. The mortality rate for interstitial EPs is 2% to 2.5% compared with only 0.14% for other tubal EPs. Risk factors for interstitial EPs are similar to those for all other EPs; however, in vitro fertilization (IVF) and previous salpingectomy are significant predisposing factors.
On US examination, interstitial EPs are diagnosed when the gestational sac is found eccentrically located within the myometrium of the uterine fundus but separate from the endometrium. Interstitial pregnancies are most easily diagnosed when myometrial tissue incompletely surrounds the gestational sac and the lateral margin protrudes beyond the serosal surface of the uterus ( Figure 22-10 ). Occasionally, however, a small interstitial gestational sac may be completely surrounded by myometrium, which will be thinned laterally, and the gestational sac will be clearly eccentric. In this situation, an interstitial pregnancy may be difficult to differentiate from a cornual pregnancy, which is a true IUP but located eccentrically within the endometrium in the cornua (horn) of a septate or bicornuate uterus. Mass effect from a fibroid or myometrial contraction can also cause an IUP to appear eccentrically located. The interstitial line sign describes a thin echogenic line, representing the interstitial portion of the fallopian tube, that extends from the margin of the intramural gestational sac to the endometrial cavity. This sign has been reported to be 80% sensitive and 98% specific for identifying interstitial EPs. In our experience, however, it is rarely observed. More commonly, what has been helpful in our practice is the observation of myometrial tissue clearly separating the edge of the gestational sac from the echogenic endometrial canal (see Figure 22-10 , B ), which confirms that the gestational sac is separate from the endometrial cavity and therefore cannot be an IUP. Three-dimensional US can also play an important role in accurately diagnosing an interstitial EP.