


Oculodermal melanocytosis (ODM), also known as nevus of Ota, is a congenital pigmentary abnormality in the periocular region characterized by excessive melanocytes. This condition is usually unilateral and classically involves the eyelids, sclera, uvea, orbit, and, less commonly, sites including the meninges, palate, and tympanic membrane.1,2
AT A GLANCE
• Oculodermal melanocytosis (ODM) is an uncommon condition, but it is a profound predisposing factor for uveal melanoma.
• ODM serves as a precursor to uveal melanoma, and recognition of ODM can allow early detection of melanoma. However, melanoma that arises in eyes with ODM carries a more ominous prognosis.
• The presence of an elevated choroidal mass, even if of minimal thickness, in an eye with choroidal ODM is suspicious, and the detection of subretinal fluid and/or orange pigment is key to detection of early-stage choroidal melanoma.
• Given the correlation between ODM and high-risk uveal melanoma, patients manifesting either diffuse or sector ODM should be thoroughly examined on a twice-yearly basis.
The prevalence of ODM in the general population is estimated to be 0.04%, but in those with uveal melanoma ODM is found in approximately 3% of patients.1,3,4 ODM serves as a precursor to uveal melanoma, a relationship that is important to understand, as recognition of ODM can allow early detection of melanoma. On the downside, however, melanoma that arises in eyes with ODM carries a more ominous prognosis. Two studies have documented that such patients exhibit double the risk for melanoma-related metastasis compared with patients with melanoma arising in an eye without melanocytosis.1,5
Recent cytogenetic investigation of uveal melanoma has revealed mutations in chromosomes 3, 6, and 8, with chromosome 3 monosomy and 8q gain associated with a 20-times greater risk for melanoma-related metastasis compared with melanoma without mutation.6,7
In a comprehensive cytogenetic analysis of 1059 patients with uveal melanoma, 8q gain was significantly associated with clinical features of ciliary body location, greater tumor thickness, and the presence of ocular melanocytosis.6 Herein we describe a patient with new-onset choroidal melanoma in an eye with previously unrecognized ODM.
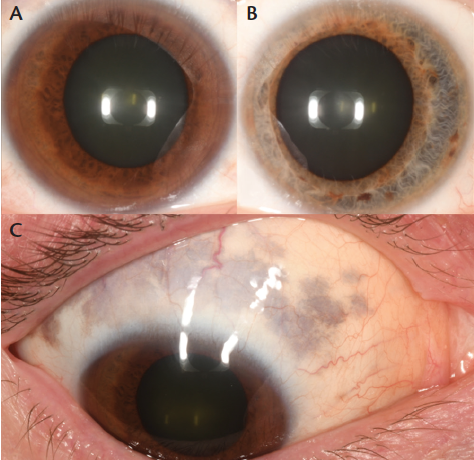
Figure 1. External photographs of a 51-year-old white man with ODM and choroidal melanoma OD at presentation. Heterochromia is evident, with dark brown iris OD (A) and blue iris OS (B). Detailed examination of the sclera revealed hyperpigmentation typical of scleral melanocytosis (C).
CASE REPORT
A 51-year-old healthy man noted chronic photopsia in his right eye (OD) for a period of 1 year. On examination elsewhere, he was found to have an intraocular mass and was referred to our center for management. He recalled having had several previous eye examinations sporadically over the years with presumed normal findings and no mention of ODM or other abnormalities.
On our examination, there was notable heterochromia, manifesting with a dark brown iris OD and a light blue iris in the left eye (OS). In addition, there was unilateral pigmentation in the periocular cutaneous region, sclera, iris, and choroid OD, suggestive of ODM (Figure 1). No pigmentation was noted OS. The patient, a physician, was not aware of his ODM or its ultimate risks, but he did realize that he demonstrated heterochromia.
The patient’s visual acuity was 20/25 OD and 20/20 OS. Intraocular pressures were normal, and examination OS was unremarkable. Evaluation of the fundus OD disclosed a lightly pigmented choroidal mass measuring 18.0 mm in diameter and 5.8 mm in thickness, extending from the ora serrata to 3 mm from the optic disc. Ultrasonography confirmed an echolucent mass with intrinsic vascular pulsations consistent with uveal melanoma (Figure 2). Subretinal fluid was observed clinically and confirmed with optical coherence tomography (OCT).
The patient was diagnosed with ODM and choroidal melanoma. Classification per the American Joint Committee on Classification 8th edition was T3a, N0, M0. The patient was treated with I-125 plaque radiotherapy using a radiation field of 22 mm for apex dose of 70 Gy.
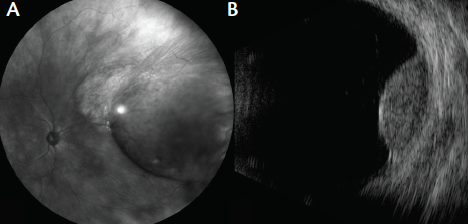
Figure 2. Wide-angle imaging of the fundus revealed choroidal melanoma in the nasal periphery OD (A). Ultrasonography confirmed a dome-shaped melanoma measuring 5.8 mm in thickness (B).
DISCUSSION
ODM is an uncommon condition, but it is a profound predisposing factor for uveal melanoma. It is estimated that one in 400 white individuals with ODM will develop uveal melanoma during his or her lifetime.2 Clinically, ODM can present with a diffuse distribution, affecting the entire globe with pigmentation, or it can manifest with a sectoral distribution, affecting only a small portion of the eye.1 Both diffuse and sectoral melanocytosis can lead to melanoma in the region of pigmentation.
Shields et al described 89 patients with sectoral ODM involving the sclera (44%), eyelid (4%), iris (65%), and choroid (54%), and the ODM involved approximately 5 to 6 clock hours of uvea.8 In this cohort, uveal melanoma was found within the ODM in seven (8%) cases.8 In some instances, the periocular dermal pigmentation and/or the slate-grey scleral pigmentation is subtle and not obvious when compared with the fellow eye. In all patients with uveal melanoma, careful inspection for ODM is advised.
A study of 7872 consecutive eyes with uveal melanoma revealed that underlying ODM was present in 230 (3%) cases.1 The rate of uveal melanoma metastasis in eyes with ODM was 1.6 times higher than in those without ODM. The relative risk for metastasis with ODM compared to without ODM relied somewhat on the tissues involved. Iris melanocytosis imparted 2.8-times greater risk, and choroidal melanocytosis a 2.6-times greater risk.1 A subsequent matched study comparing uveal melanoma metastatic risk found that eyes with ODM were overall twice as likely to have systemic metastasis compared to eyes without ODM.5
In two recent large studies, investigators studied the relationships of cytogenetics to clinical features and to systemic prognosis.6,7 In one study, researchers observed that eyes with ODM showed a significantly higher rate of mutation in chromosome 8 (8q gain; odds ratio, 2.75), and this mutation portended 20 times greater risk for metastatic disease.6 The other study calculated Kaplan-Meier estimates for metastasis based on various cytogenetic signatures and found that the risk of melanoma metastasis in patients with 8q gain at 3, 5, and 7 years was 21%, 35%, and 48%, respectively—greater than for patients with no mutation at 2%, 7%, and 7%, at the same intervals respectively.7
CONCLUSION
The relationship between ODM and the development of uveal melanoma is an important one to recognize. Given the correlation between ODM and high-risk uveal melanoma, patients manifesting either diffuse or sectoral ODM should be thoroughly examined on a twice-yearly basis.1 When examining an eye with choroidal ODM, the presence of an elevated choroidal mass, even if of minimal thickness, is suspicious, and the detection of subretinal fluid and/or orange pigment (lipofuscin) is key to the detection of early-stage choroidal melanoma.
It is quite important to note that multimodal testing can assist with recognition of these often subtle features. OCT can be used to detect subretinal fluid, and fundus autofluorescence can be used for lipofuscin.9 In the case described above, both subretinal fluid and orange pigment were present. Comprehensive examinations are vital to early detection, treatment, and better prognosis.
1. Shields CL, Kaliki S, Livesey M, et al. Association of ocular and oculodermal melanocytosis with the rate of uveal melanoma metastasis: analysis of 7872 consecutive eyes. JAMA Ophthalmol. 2013;131(8):993-1003.
2. Singh AD, De Potter P, Fijal BA, et al. Lifetime prevalence of uveal melanoma in white patients with oculo(dermal) melanocytosis. Ophthalmology. 1998;105(1):195-198.
3. Gonder JR, Ezell PC, Shields JA, Augsburger JJ. Ocular melanocytosis: A study to determine the prevalence rate of ocular melanocytosis. Ophthalmology. 1982;89(8):950-952.
4. Gonder JR, Shields JA, Augsburger JJ. Uveal malignant melanoma associated with ocular and oculodermal melanocytosis. Ophthalmology. 1982;89(8):953-960.
5. Mashayekhi A, Kalkiki S, Walker B, et al. Metastasis from uveal melanoma associated with congenital melanocytosis. A matched study. Ophthalmology. 2013;120(7):1265-1268.
6. Shields CL, Say EAT, Hasanreisoglu M, et al. Cytogenetic abnormalities in uveal melanoma based on tumor features and size in 1059 patients: The 2016 W. Richard Green Lecture. Ophthalmology. 2017;124(5):609-618.
7. Shields CL, Say EAT, Hasanreisoglu M, et al. Personalized prognosis of uveal melanoma based on cytogenetic profile in 1059 patients over an 8-year period: The 2017 Harry S. Gradle Lecture [published online ahead of print May 7, 2017]. Ophthalmology.
8. Shields CL, Qureshi A, Mashayekhi A, et al. Sector (partial) oculo(dermal) melanocytosis in 89 eyes. Ophthalmology. 2011;118(12):2474-2479.
9. Shields JA, Shields CL. Congenital oculo(dermal) melanocytosis. In: Shields JA, Shields CL, eds. Intraocular Tumors: A Textbook and Atlas. Philadelphia, PA: Lippincott, William & Wilkins; 2008:6-12.
Luis A. Acaba-Berrocal, BA
• medical student at the Sidney Kimmel Medical College at Thomas Jefferson University, Philadelphia, Pa.
Vladislav P. Bekerman, BS
• medical student at Drexel University College of medicine and a graduate student researcher at the Ocular Oncology Service, Wills Eye Hospital, Thomas Jefferson University, Philadelphia, Pa.
Carol L. Shields, MD
• director of the Ocular Oncology Service, Wills Eye Hospital, Thomas Jefferson University, Philadelphia, Pa.
• member of the Retina Today editorial advisory board
• carolshields@gmail.com
No conflicting relationship exists for any author.
Support provided by the Eye Tumor Research Foundation, Philadelphia, Pa. (CLS). The funders had no role in the design and conduct of the study, in the collection, analysis, and interpretation of the data, or in the preparation, review, or approval of the manuscript. Carol L. Shields, MD, has had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.


















