To the Editor:
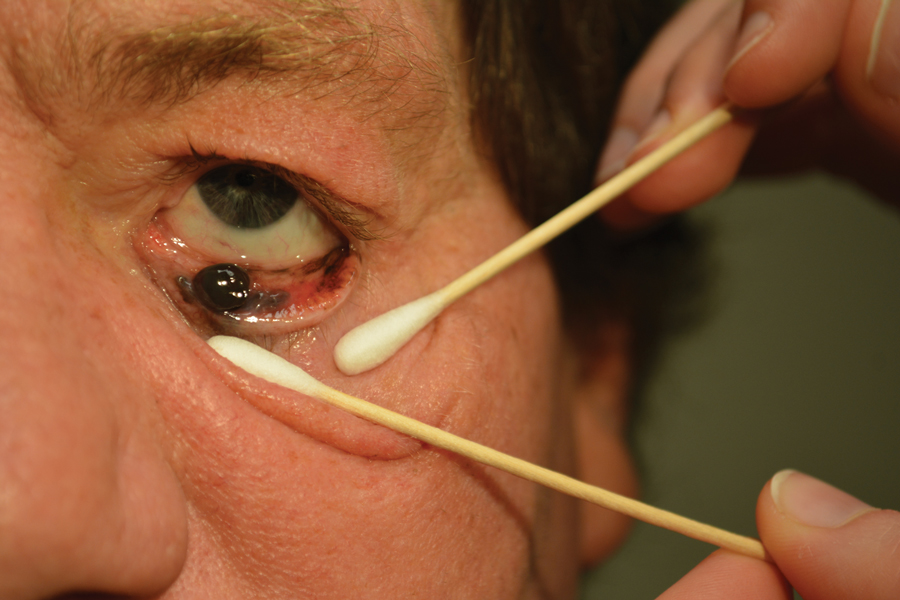
A 58-year-old man with a pigmented lesion on the left lower eyelid was referred to the oculoplastic clinic by an outside ophthalmologist. The patient had noticed the lesion growing over the course of the last 4 to 5 months. He reported scant amounts of blood and discharge coming from the nose and left eye the week prior, which persisted for 3 days. He had no associated pain or discomfort. A slit-lamp examination revealed a pigmented left lower eyelid lesion measuring 20×15 mm with telangiectasia and an eyelid margin abnormality with no palpable lymphadenopathy. The patient was diagnosed with clinical stage T3N0M0 malignant conjunctival melanoma of the left eyelid based on the American Joint Committee on Cancer classification. It is thought to have originated from conjunctival primary acquired melanosis (PAM). The T3 stage is defined as malignant melanoma with local invasion; the lesion involved the eyelid and puncta as well as canalicular portions of the lacrimal drainage system.1 The bloody discharge was attributed to the involvement of the canalicular system, which drains tears from the eye to the nose. Melanomas can bleed, so any bloody discharge from the eye also will come through the ipsilateral nasal passage. Oncology evaluated the lesion to help determine the stage, and they found no lymph node involvement or brain, neck, chest, abdominal, or pelvic metastasis by computed tomography and magnetic resonance imaging. Sentinel lymph node biopsy was discussed with head and neck oncology specialists and was ultimately not performed per the recommendation from the Head and Neck Oncology Board; it is not a common modality for managing conjunctival melanoma because it has not been shown to alter morbidity and mortality.
The entire eyelid from the medial canthus to the lateral canthus was removed without touching the pigmented mass to ensure a “no-touch” technique removal of the mass. The no-touch technique primarily is utilized to decrease the likelihood of instrumental seeding of healthy tissues or the vascular system.2 This technique focuses on preventing any direct manipulation of the tumor and avoiding an incisional biopsy as well as removal of the tumor en bloc. The margins were cutaneous—3 mm lateral to the lateral canthus, 5 mm below the lid margin, and 3 mm medial to the medial canthus—with dissection of the medial tissue from the orbital rim and lacrimal sac fossa. The lacrimal sac and lower canaliculus were then resected. The conjunctiva 5 mm inferior to the pigmented mass and the entire palpebral conjunctiva was resected to the inferior fornix across the entire palpebral conjunctiva of the lower eyelid (Figure). The eyelid and lacrimal portions were removed as a unit. Essentially, the entire lower eyelid (full thickness), including the lateral canthus, medial canthus, canaliculus, and lacrimal sac, were removed en bloc. The final tumor staging after tissue evaluation by pathology and systemic evaluation by oncology was pT3N0bM0.
A tarsoconjunctival (Hughes) flap from the upper eyelid was used to reconstruct the posterior lamella (tarsus/conjunctiva) of the lower eyelid, and a full-thickness skin graft harvested from the ipsilateral upper eyelid was used to reconstruct the anterior lamella (skin) of the lower eyelid. The reconstruction site was allowed to heal for 4 weeks before severing the tarsoconjunctival graft to allow the separation of the upper and lower eyelids. Adjunctive topical ophthalmic chemotherapy (mitomycin C 0.04%) was started 4 weeks after the last surgery. The medication was applied 4 times daily for 1 week and restarted after the conjunctival erythema and injection subsided, which was approximately 2.5 weeks, on average. The regimen of applying the medication (1 week on and 2.5 weeks off) was completed for 4 cycles. At 1 year follow-up after his diagnosis, the patient was without local recurrence or evidence of systemic metastasis. We plan to have him continue ophthalmic and oncologic evaluation every 3 to 4 months for the next 24 months, and then every 6 months for years 2 through 5.
Ocular melanoma can be further divided into uveal and conjunctival types, both arising from the respective tissue. Melanoma of the conjunctiva commonly arises from PAM with atypia, which is an acquired conjunctival pigmented lesion similar to a skin nevus that has the potential to become dysplastic. In a genetic analysis of 78 conjunctival melanomas, BRAF mutations were identified in 29% (23/78) of tumors, and NRAS mutations were detected in 18% (14/78) of tumors3; however, in our case, there were no BRAF or NRAS mutations detected. In a study of 84,836 cases that included a diagnosis of melanoma, ocular melanoma comprised 5.2% of melanomas, with cutaneous, mucosal, and unknown primary sites totaling the remaining percentage of melanomas. Of 4522 patients with ocular melanomas, 85% had uveal melanomas; 4.8% had conjunctival melanoma; and 10.2% were classified as other—comprised of cornea, not otherwise specified (NOS); retina; lacrimal gland; orbit, NOS; overlapping lesion of the eye; and eye, NOS.4 Melanomas of the uvea, including the ciliary body, choroid, and iris, result from a notably different pathogenesis than conjunctival melanoma, with the former being primarily associated with GNAQ and GNA mutations.3 Ciliary body and choroidal melanomas each have a different pathogenesis for occurrence, with choroidal melanoma being mostly from metastasis and ciliary body melanoma from mutations or metastasis.
Pigmented lesions on the conjunctiva or sclera arise from either melanocytes or nonmelanocytes and have a diverse differential diagnosis, including congenital melanosis, conjunctival nevi, PAM or secondary acquired melanosis, or conjunctival melanoma. The diagnosis of uveal melanoma should be based on fundoscopic examination by an experienced clinician. Uveal melanoma is unlike most other cancers in that diagnosis can be by clinical fundoscopic examination alone. Imaging studies such as ultrasound and fluorescein angiography can be performed for prognostication and characterization. Fine needle aspiration biopsy for molecular analysis is becoming more routine, but the results rarely affect the plan of care. Primary treatment of uveal melanoma should strive to preserve vision and prevent metastasis; however, a primary modality has yet to show notable results in decreasing distant disease spread or overall survival. Treatment of the primary tumor should involve consideration of tumor size, location, health of the patient, and patient preference.1,5
For patients with melanoma arising from the conjunctiva, initial management should focus on local disease control, including wide local excision to avoid seeding, supplemented with cryotherapy and alcohol epitheliectomy to the cornea to ensure local tumor extinction.2,6 Techniques including enucleation and orbital exenteration historically have been used for treatment of extensive disease, but this approach has not been associated with improvement in mortality and is a cause of notable morbidity.7,8 Sentinel lymph node biopsy has an established role in the management of cutaneous melanoma, but its use in the setting of conjunctival melanoma is controversial, with studies showing that up to 50% of patients with local recurrence can develop distant metastasis with no evidence of regional lymph node involvement.9,10 When the tumor is present at the surgical margins or in the case that lesions cannot be fully excised, adjuvant therapy may improve long-term control and prevent recurrence following surgical intervention. Mitomycin C 0.04% is the most commonly used topical chemotherapy agent because it has an established role in the treatment of PAM, but it remains adjuvant therapy for conjunctival melanoma due to the relatively poor outcomes when it is used for primary therapy.11
In one study, recurrence rates for conjunctival melanoma were 26%, 51%, and 65% at 5, 10, and 15 years, respectively.12 Risk factors for recurrence include increased tumor thickness, incomplete excision, positive margins, surgical excision without adjuvant therapy, and nonlimbal location.13 A multivariate analysis of 150 patients showed that the melanoma location not touching the limbus (P=.01) and pathologic evidence of tumor to the lateral margin (P=.02) were related to tumor recurrence, with relative risks (IQR) of 2.3 (1.2-4.6) and 2.9 (1.2-7.1), respectively. Careful surgical planning using wide microsurgical excisional biopsy emphasizing a no-touch technique as well as supplemental alcohol therapy for the cornea and conjunctiva is advised.12