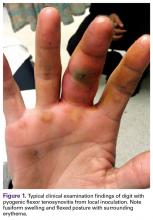
Pyogenic flexor tenosynovitis (PFT) is a common closed space infection of the flexor tendon sheaths of the hand and remains one of the most challenging problems encountered in orthopedic and hand surgery (Figure 1). PFT also is known as septic flexor tenosynovitis and suppurative flexor tenosynovitis.
Kanavel1 initially described 4 cardinal signs that characterize infection of the flexor tendon sheath: symmetric fusiform swelling of the entire digit, exquisite tenderness to palpation along the course of the tendon sheath, semiflexed posture at rest, and pain with attempted passive extension of the digit. The prevalence of this infection ranges from 2.5% to 9.4%.2 Once the infection is established in a patient, it can cause significant morbidity and disability and produce an economic burden. It can also present a significant treatment dilemma for the treating surgeon, as there is no standardized protocol for managing this common but challenging hand infection. For treatment, many surgeons combine surgical decompression, sheath irrigation, and empiric intravenous (IV) antibiotic administration. However, despite prompt treatment, and regardless of the protocol used, complication rates as high as 38% have been reported.3 Moreover, even after infection eradication, a significant proportion of patients continue to have pain, swelling, stiffness, loss of composite flexion, weakness, and recurrence that potentially requires amputation.
1. What Causes Pyogenic Flexor Tenosynovitis?
PFT can result from hematogenous spread, but local inoculation by a laceration, a puncture, or a bite also is common4-7 (Figure 1). As a consequence of these mechanisms of injury, the most common source of PFT is skin flora. Staphylococcus aureus has been found in up to 75% of positive cultures in several studies.2,5,6,8,9 Methicillin-resistant S aureus (MRSA) has been found in up to 29% of cases, and the incidence continues to increase, particularly in urban areas.2,9-12 Other common bacteria are Staphylococcus epidermidis, β-hemolytic Streptococcus species, and Pseudomonas aeruginosa.5,6,10 Infection by more than 1 species of bacteria is also fairly prevalent. Of 62 patients in a study, 38% had infections with 1 organism, and 62% with 2 or more.6 Twenty-six percent of cultures grew mixed anaerobic and aerobic organisms.6 PFT is seldom caused by Eikenella corrodens from a human bite or Pasteurella multocida from an animal bite.10 Other rare causes of PFT are Listeria monocytogenes13 and Clostridium difficile from a gastrointestinal source.14Neisseria gonorrhea can cause acute tenosynovitis, usually in the setting of disseminated gonococcal infection.15,16 Also reported is mycobacterial tenosynovitis, most commonly caused by Mycobacterium kansasii and Mycobacterium marinum.17
2. Which Antibiotics Are Best Suited to Empirical Management of PFT?
Management of PFT, regardless of the pathogen, includes prompt administration of empiric IV antibiotics, usually followed by surgical drainage.7,18-20 While cultures are being tested, antibiotics should be selected—including antibiotics for empiric coverage against common gram-positive organisms, including Staphylococcus and Streptococcus species.12 The Centers for Disease Control and Prevention recommends empiric coverage for MRSA if the local prevalence exceeds 10% to 15%. Recommended empiric antibiotics are trimethoprim-sulfamethoxazole (TMP-SMX) and clindamycin (both oral) and clindamycin, vancomycin, and daptomycin (all IV).
In addition, institutional and local antibiotic resistance patterns of bacteria should guide treatment and antibiotic selection. First-generation cephalosporins have long been the cornerstone of treatment for infections caused by S aureus, but increasing methicillin resistance has reduced their role in the treatment, particularly the empiric treatment, of MRSA infections. Methicillin resistance first appeared as nosocomial S aureus infections in 1961, only 1 year after the introduction of the semisynthetic penicillin class that includes methicillin. Over the past 2 decades, MRSA has emerged in the community in otherwise young and healthy individuals with no healthcare-associated risk factors. Fortunately, several readily available antibiotics have maintained their efficacy in managing these “community-acquired” MRSA hand infections. TMP-SMX provides adequate coverage for MRSA and is a relatively inexpensive medication, and clindamycin is an equally effective and cost-effective alternative.
Presumptive antibiotics should also cover gram-negative rods and anaerobes, including Clostridium species, especially in immunocompromised patients.7,9 These patients may require additional antibiotics for presumptive coverage of other rarer bacterial causes, especially when unique mechanisms of injury (eg, aquatic injury, farm injury) are involved. Once culture results are ready, antibiotic regimens should be narrowed to cover the specific organisms identified.