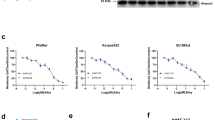
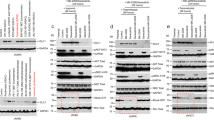
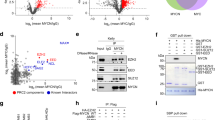
Abstract
Activating mutations in EZH2, the catalytic component of PRC2, promote cell proliferation, tumorigenesis, and metastasis through enzymatic or non-enzymatic activity. The EZH2-Y641 gain-of-function mutation is one of the most significant in diffuse large B-cell lymphoma (DLBCL). Although EZH2 kinase inhibitors, such as EPZ-6438, provide clinical benefit, certain cancer cells are resistant to the enzymatic inhibition of EZH2 because of the inability to functionally target mutant EZH2, or because of cells’ dependence on the non-histone methyltransferase activity of EZH2. Consequently, destroying mutant EZH2 protein may be more effective in targeting EZH2 mutant cancers that are dependent on the non-catalytic activity of EZH2. Here, using extensive selectivity profiling, combined with genetic and animal model studies, we identified USP47 as a novel regulator of mutant EZH2. Inhibition of USP47 would be anticipated to block the function of mutated EZH2 through induction of EZH2 degradation by promoting its ubiquitination. Moreover, targeting of USP47 leads to death of mutant EZH2-positive cells in vitro and in vivo. Taken together, we propose targeting USP47 with a small molecule inhibitor as a novel potential therapy for DLBCL and other hematologic malignancies characterized by mutant EZH2 expression.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Czermin B, Melfi R, McCabe D, Seitz V, Imhof A, Pirrotta V. Drosophila enhancer of Zeste/ESC complexes have a histone H3 methyltransferase activity that marks chromosomal Polycomb sites. Cell. 2002;111:185–96.
Margueron R, Reinberg D. The Polycomb complex PRC2 and its mark in life. Nature. 2011;469:343–9.
Liu X, Wu Q, Li L. Functional and therapeutic significance of EZH2 in urological cancers. Oncotarget. 2017;8:38044–55.
Kim KH, Roberts CW. Targeting EZH2 in cancer. Nat Med. 2016;22:128–34.
Lunning MA, Green MR. Mutation of chromatin modifiers; an emerging hallmark of germinal center B-cell lymphomas. Blood cancer J. 2015;5:e361.
Varambally S, Dhanasekaran SM, Zhou M, Barrette TR, Kumar-Sinha C, Sanda MG, et al. The polycomb group protein EZH2 is involved in progression of prostate cancer. Nature. 2002;419:624–9.
Sashida G, Iwama A. Multifaceted role of the polycomb-group gene EZH2 in hematological malignancies. Int J Hematol. 2017;105:23–30.
McCabe MT, Ott HM, Ganji G, Korenchuk S, Thompson C, Van Aller GS, et al. EZH2 inhibition as a therapeutic strategy for lymphoma with EZH2-activating mutations. Nature. 2012;492:108–12.
Stasik S, Middeke JM, Kramer M, Röllig C, Krämer A, Scholl S, et al. EZH2 mutations and impact on clinical outcome: an analysis in 1,604 patients with newly diagnosed acute myeloid leukemia. Haematologica. 2020;105:e228–e231.
Mechaal A, Menif S, Abbes S, Safra I. EZH2, new diagnosis and prognosis marker in acute myeloid leukemia patients. Adv Med Sci. 2019;64:395–401.
Xu K, Wu ZJ, Groner AC, He HH, Cai C, Lis RT, et al. EZH2 oncogenic activity in castration-resistant prostate cancer cells is Polycomb-independent. Science. 2012;338:1465–9.
Kim KH, Kim W, Howard TP, Vazquez F, Tsherniak A, Wu JN, et al. SWI/SNF-mutant cancers depend on catalytic and non-catalytic activity of EZH2. Nat Med. 2015;21:1491–6.
Wu S, Fatkhutdinov N, Fukumoto T, Bitler BG, Park PH, Kossenkov AV, et al. SWI/SNF catalytic subunits’ switch drives resistance to EZH2 inhibitors in ARID1A-mutated cells. Nat Commun. 2018;9:4116.
Viré E, Brenner C, Deplus R, Blanchon L, Fraga M, Didelot C, et al. The Polycomb group protein EZH2 directly controls DNA methylation. Nature. 2006;439:871–4.
Schuettengruber B, Chourrout D, Vervoort M, Leblanc B, Cavalli G. Genome regulation by polycomb and trithorax proteins. Cell. 2007;128:735–45.
Cao R, Wang L, Wang H, Xia L, Erdjument-Bromage H, Tempst P, et al. Role of histone H3 lysine 27 methylation in Polycomb-group silencing. Science. 2002;298:1039–43.
Morin RD, Johnson NA, Severson TM, Mungall AJ, An J, Goya R, et al. Somatic mutations altering EZH2 (Tyr641) in follicular and diffuse large B-cell lymphomas of germinal-center origin. Nat Genet. 2010;42:181–5.
Dubois S, Mareschal S, Picquenot J-M, Viailly P-J, Bohers E, Cornic M, et al. Immunohistochemical and genomic profiles of diffuse large B-cell lymphomas: Implications for targeted EZH2 inhibitor therapy? Oncotarget. 2015;6:16712–24.
Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29.
Batra R, Kaur H, Jindal S. Extranodal large B-cell type aggressive non-Hodgkin’s lymphoma. Indian J Dent. 2014;5:225–8.
Fan L, Li L, Zhou Y, Li J. Rituximab-based therapy in newly diagnosed diffuse large B-Cell Lymphoma patients: Individualized risk-adapted therapy approach using molecular subtypes. J Hematol. 2017;6:33–43.
Held G, Pöschel V, Pfreundschuh M. Rituximab for the treatment of diffuse large B-cell lymphomas. Expert Rev anticancer Ther. 2006;6:1175–86.
Bödör C, O’Riain C, Wrench D, Matthews J, Iyengar S, Tayyib H, et al. EZH2 Y641 mutations in follicular lymphoma. Leukemia. 2011;25:726–9.
Sneeringer CJ, Scott MP, Kuntz KW, Knutson SK, Pollock RM, Richon VM, et al. Coordinated activities of wild-type plus mutant EZH2 drive tumor-associated hypertrimethylation of lysine 27 on histone H3 (H3K27) in human B-cell lymphomas. Proc Natl Acad Sci USA. 2010;107:20980–5.
Yap DB, Chu J, Berg T, Schapira M, Cheng SW, Moradian A, et al. Somatic mutations at EZH2 Y641 act dominantly through a mechanism of selectively altered PRC2 catalytic activity, to increase H3K27 trimethylation. Blood. 2011;117:2451–9.
Venney D, Mohd-Sarip A, Mills KI. The impact of epigenetic modifications in myeloid malignancies. Int J Mol Sci. 2021; 9: 22.
Nikoloski G, Langemeijer SM, Kuiper RP, Knops R, Massop M, Tönnissen ER, et al. Somatic mutations of the histone methyltransferase gene EZH2 in myelodysplastic syndromes. Nat Genet. 2010;42:665–7.
Bejar R, Stevenson K, Abdel-Wahab O, Galili N, Nilsson B, Garcia-Manero G, et al. Clinical effect of point mutations in myelodysplastic syndromes. N. Engl J Med. 2011;364:2496–506.
Béguelin W, Popovic R, Teater M, Jiang Y, Bunting KL, Rosen M, et al. EZH2 is required for germinal center formation and somatic EZH2 mutations promote lymphoid transformation. Cancer Cell. 2013;23:677–92.
Knutson SK, Kawano S, Minoshima Y, Warholic NM, Huang KC, Xiao Y, et al. Selective inhibition of EZH2 by EPZ-6438 leads to potent antitumor activity in EZH2-mutant non-Hodgkin lymphoma. Mol Cancer Therapeutics. 2014;13:842–54.
Italiano A. Targeting epigenetics in sarcomas through EZH2 inhibition. J Hematol Oncol. 2020;13:33–33.
Kurmasheva RT, Sammons M, Favours E, Wu J, Kurmashev D, Cosmopoulos K, et al. Initial testing (stage 1) of tazemetostat (EPZ-6438), a novel EZH2 inhibitor, by the Pediatric Preclinical Testing Program. Pediatr Blood Cancer 2017; 64: https://doi.org/10.1002/pbc.26218.
Huang S, Wang Z, Zhou J, Huang J, Zhou L, Luo J, et al. EZH2 inhibitor GSK126 suppresses antitumor immunity by driving production of myeloid-derived suppressor cells. Cancer Res. 2019;79:2009.
Yap TA, Winter JN, Giulino-Roth L, Longley J, Lopez J, Michot JM, et al. Phase I study of the novel enhancer of zeste homolog 2 (EZH2) inhibitor GSK2816126 in patients with advanced hematologic and solid tumors. Clin Cancer Res. 2019;25:7331–9.
Lee ST, Li Z, Wu Z, Aau M, Guan P, Karuturi RK, et al. Context-specific regulation of NF-κB target gene expression by EZH2 in breast cancers. Mol cell. 2011;43:798–810.
Gibaja V, Shen F, Harari J, Korn J, Ruddy D, Saenz-Vash V, et al. Development of secondary mutations in wild-type and mutant EZH2 alleles cooperates to confer resistance to EZH2 inhibitors. Oncogene. 2016;35:558–66.
Li Z, Hou P, Fan D, Dong M, Ma M, Li H, et al. The degradation of EZH2 mediated by lncRNA ANCR attenuated the invasion and metastasis of breast cancer. Cell Death Differ. 2017;24:59–71.
Zhang P, Xiao Z, Wang S, Zhang M, Wei Y, Hang Q, et al. ZRANB1 Is an EZH2 deubiquitinase and a potential therapeutic target in breast cancer. Cell Rep. 2018;23:823–37.
Wilkinson KD. Ubiquitination and deubiquitination: Targeting of proteins for degradation by the proteasome. Seminars Cell Developmental Biol. 2000;11: 141–8.
D’Arcy P, Wang X, Linder S. Deubiquitinase inhibition as a cancer therapeutic strategy. Pharm Ther. 2015;147:32–54.
Fraile JM, Quesada V, Rodriguez D, Freije JM, Lopez-Otin C. Deubiquitinases in cancer: new functions and therapeutic options. Oncogene. 2012;31:2373–88.
Park JM, Lee JE, Park CM, Kim JH. USP44 promotes the tumorigenesis of prostate cancer cells through EZH2 protein stabilization. Mol Cells. 2019;42:17–27.
Chen Y, Zhou B, Chen D. USP21 promotes cell proliferation and metastasis through suppressing EZH2 ubiquitination in bladder carcinoma. Onco Targets Ther. 2017;10:681–9.
Zheng N, Chu M, Lin M, He Y, Wang Z. USP7 stabilizes EZH2 and enhances cancer malignant progression. Am J Cancer Res. 2020;10:299–313.
Wigle TJ, Knutson SK, Jin L, Kuntz KW, Pollock RM, Richon VM, et al. The Y641C mutation of EZH2 alters substrate specificity for histone H3 lysine 27 methylation states. FEBS Lett. 2011;585:3011–4.
Messingerova L, Imrichova D, Kavcova H, Turakova K, Breier A, Sulova Z. Acute myeloid leukemia cells MOLM-13 and SKM-1 established for resistance by azacytidine are crossresistant to P-glycoprotein substrates. Toxicol Vitr. 2015;29:1405–15.
Lei H, Xu H-Z, Shan H-Z, Liu M, Lu Y, Fang Z-X, et al. Targeting USP47 overcomes tyrosine kinase inhibitor resistance and eradicates leukemia stem/progenitor cells in chronic myelogenous leukemia. Nature. Communications. 2021;12:51.
Yang J, Weisberg EL, Liu X, Magin RS, Chan WC, Hu B, et al. Small molecule inhibition of deubiquitinating enzyme JOSD1 as a novel targeted therapy for leukemias with mutant JAK2. Leukemia 2021. [Online ahead of print].
Bisserier M, Wajapeyee N. Mechanisms of resistance to EZH2 inhibitors in diffuse large B-cell lymphomas. Blood. 2018;131:2125–37.
Zhang Q, Han Q, Zi J, Ma J, Song H, Tian Y, et al. Mutations in EZH2 are associated with poor prognosis for patients with myeloid neoplasms.Genes Dis. 2019;6:276–81.
Kasinath V, Faini M, Poepsel S, Reif D, Feng XA, Stjepanovic G, et al. Structures of human PRC2 with its cofactors AEBP2 and JARID2. Science. 2018;359:940–4.
Jiao L, Liu X. Structural basis of histone H3K27 trimethylation by an active polycomb repressive complex 2. Science. 2015;350:aac4383.
Højfeldt JW, Laugesen A, Willumsen BM, Damhofer H, Hedehus L, Tvardovskiy A, et al. Accurate H3K27 methylation can be established de novo by SUZ12-directed PRC2. Nat Struct Mol Biol. 2018;25:225–32.
Yamaguchi H, Hung MC. Regulation and role of EZH2 in cancer. Cancer Res Treat. 2014;46:209–22.
Zhou J, Huang S, Wang Z, Huang J, Xu L, Tang X, et al. Targeting EZH2 histone methyltransferase activity alleviates experimental intestinal inflammation. Nat Commun. 2019;10:2427.
Duan R, Du W, Guo W. EZH2: a novel target for cancer treatment. J Hematol Oncol. 2020;13:104.
Zee BM, Levin RS, Xu B, LeRoy G, Wingreen NS, Garcia BA. In vivo residue-specific histone methylation dynamics. J Biol Chem. 2010;285:3341–50.
Waterborg JH. Dynamic methylation of alfalfa histone H3. J Biol Chem. 1993;268:4918–21.
Altun M, Kramer Holger B, Willems Lianne I, McDermott Jeffrey L, Leach Craig A, Goldenberg Seth J, et al. Activity-based chemical proteomics accelerates inhibitor development for deubiquitylating enzymes. Chem Biol. 2011;18:1401–12.
Weinstock J, Wu J, Cao P, Kingsbury WD, McDermott JL, Kodrasov MP, et al. Selective dual inhibitors of the cancer-related deubiquitylating proteases USP7 and USP47. ACS medicinal Chem Lett. 2012;3:789–92.
Schauer NJ, Liu X, Magin RS, Doherty LM, Chan WC, Ficarro SB, et al. Selective USP7 inhibition elicits cancer cell killing through a p53-dependent mechanism. Sci Rep. 2020;10:5324.
Wang X, Cao W, Zhang J, Yan M, Xu Q, Wu X, et al. A covalently bound inhibitor triggers EZH2 degradation through CHIP-mediated ubiquitination. Embo J. 2017;36:1243–60.
Yang SW, Oh KH, Park E, Chang HM, Park JM, Seong MW, et al. USP47 and C Terminus of Hsp70-Interacting Protein (CHIP) antagonistically regulate Katanin-p60-mediated axonal growth. J Neurosci. 2013;33:12728.
Lei H, Ma C, Liu Z, Gao S, Zhou L, Wang W, et al. USP47 is a new target in chronic myelogenous leukemia. Blood. 2015;126:1572.
Li C, Wang Y, Gong Y, Zhang T, Huang J, Tan Z, et al. Finding an easy way to harmonize: A review of advances in clinical research and combination strategies of EZH2 inhibitors. Clin Epigenetics. 2021;13:62.
Gulati N, Béguelin W, Giulino-Roth L. Enhancer of zeste homolog 2 (EZH2) inhibitors. Leuk Lymphoma. 2018;59:1574–85.
Tiffen JC, Gunatilake D, Gallagher SJ, Gowrishankar K, Heinemann A, Cullinane C, et al. Targeting activating mutations of EZH2 leads to potent cell growth inhibition in human melanoma by derepression of tumor suppressor genes. Oncotarget. 2015;6:27023–36.
Wang Y-C, Wu Y-S, Hung C-Y, Wang S-A, Young M-J, Hsu T-I, et al. USP24 induces IL-6 in tumor-associated microenvironment by stabilizing p300 and β-TrCP and promotes cancer malignancy. Nat Commun. 2018;9:3996.
Ovaa H, Kessler BM, Rolén U, Galardy PJ, Ploegh HL, Masucci MG. Activity-based ubiquitin-specific protease (USP) profiling of virus-infected and malignant human cells. Proc Natl Acad Sci USA. 2004;101:2253.
Acknowledgements
We thank Nathanael Gray for his generous provision of reagents and technical support, and Lucia Cabal-Hierro at Dana-Farber Cancer Institute—Harvard Medical School for valuable guidance for the CRISPR-CAS9 KO assay. Our work was supported and founded by National Natural Science Foundation of China (Grant Nos. 82104198, 81903659, 32171479), Natural Science Foundation of Anhui Province (Grant No. 1908085MH259), Leukemia & Lymphoma Society’s New Idea Award, Claudia Adams Barr Award, MPN Research Foundation, Gabrielle’s Angel Foundation, NIH Foundation R01 (CA211681) and 5 P50 CA206963-02.
Author information
Authors and Affiliations
Contributions
JY, EW, and SQ conceptualized, designed, and performed the studies. JY, EW, and SQ carried out data analyses and wrote the papers. WN performed the KD assays and westerns in DLBCL. HM performed the cell viability assays and westerns. ZW helped with the generation of lentivirus particles and site mutagenesis assay. CM helped with cell culturing and western blotting. SZ assisted with shRNA KD/KO studies. MH helped with cell viability assays and westerns. ZQ and AW provided valuable scientific feedback and guidance. YJ synthesized compounds for animal studying. ZJ provided human PBMCs. TH provided human AML and DLBCL patient samples. QL measured the concentration of the compound in tissue. RM provided valuable scientific feedback. LD helped with qPCR assays. WW and JL provided valuable scientific feedback and guidance. SB, QL and JG conceptualized and designed the studies, carried out data analyses, and wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yang, J., Weisberg, E.L., Qi, S. et al. Inhibition of the deubiquitinating enzyme USP47 as a novel targeted therapy for hematologic malignancies expressing mutant EZH2. Leukemia 36, 1048–1057 (2022). https://doi.org/10.1038/s41375-021-01494-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41375-021-01494-w
This article is cited by
-
Structural and functional characterization of USP47 reveals a hot spot for inhibitor design
Communications Biology (2023)