Published online Nov 26, 2020. doi: 10.12998/wjcc.v8.i22.5663
Peer-review started: June 9, 2020
First decision: September 24, 2020
Revised: October 4, 2020
Accepted: October 26, 2020
Article in press: October 26, 2020
Published online: November 26, 2020
Oral mucositis is often observed with graft-versus-host disease (GVHD); however, the occurrence of oral granuloma is rare. The rapid increase in granulomatous lesions should be distinguished from malignant tumors in patients with GVHD because malignant diseases can develop in those patients. This case is the youngest pediatric patient with granuloma associated with GVHD.
The patient was a 1-year and 5-mo-old girl who presented to our department for the management of oral nodules. At the age of 5 mo, she was diagnosed with primary immunodeficiency disease, cord blood transplant was performed at 11 mo and bone marrow transplant at 1 year of age. After transplantation, GVHD and oral mucositis developed, and tacrolimus was administered. Interestingly, nodules appeared on the lower lip and buccal mucosa, which spontaneously disappeared. Then, a new nodule appeared on the left lateral border of the tongue. Resection was performed and the histopathological diagnosis was granuloma. The origin of these nodules were considered to be the fibroblasts activated under inflammation caused by GVHD because the calcineurin inhibitor tacrolimus acted on their proliferation.
It is very important to distinguish oral granulomatous lesions from malignancies if GVHD is present at the base and if immunosuppressive agents and steroids are being administered.
Core Tip: Oral mucositis is often observed with graft-versus-host disease (GVHD); however, the presence of oral granuloma is rare. This case is the youngest pediatric patient with granuloma associated with GVHD. At the age of 5 mo, she was diagnosed with primary immunodeficiency disease. After transplantation, she developed GVHD and oral mucositis. Notably, nodules appeared on the lip and buccal mucosa, which spontaneously disappeared. Then, a new nodule appeared on the tongue. Histopathological diagnosis was granuloma. It is important to distinguish rapidly grown granulomatous nodules from malignant tumors.
- Citation: Uesugi A, Tsushima F, Kodama M, Kuroshima T, Sakurai J, Harada H. Oral granuloma in a pediatric patient with chronic graft-versus-host disease: A case report. World J Clin Cases 2020; 8(22): 5663-5669
- URL: https://www.wjgnet.com/2307-8960/full/v8/i22/5663.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i22.5663
Although hematopoietic stem cell transplantation can be used to treat and prevent complications caused by primary immunodeficiency disease (PID), graft-versus-host disease (GVHD) can complicate recovery. Oral lesions are frequently observed in association with GVHD, typical findings include lichen planus-like mucosal changes, damage to the salivary glands, and trismus due to sclerosis. However, the occurrence of oral granuloma with GVHD is rare. Here, we report the case of a pediatric patient who developed GVHD after bone marrow transplantation (BMT) for PID and subsequently developed granuloma in the oral mucosa.
A 1-year and 5-moh-old girl presented to our department for the management of oral nodules.
At 5 mo of age, the patient was diagnosed with PID upon sustaining a severe respiratory syncytial virus infection. At 11 mo of age, she received umbilical cord blood transplant but developed hemophagocytic syndrome, and the transplant failed to engraft. At 1 year of age, BMT was performed using the bone marrow from her HLA semi-matched father. In preparation for receiving BMT, she received fludarabine (× 3 d), melphalan (× 1 d), anti-thymocyte globulin (× 2 d), and etoposide (× 2 d beginning at 3 d prior to the scheduled procedure). In an effort to prevent GVHD, tacrolimus (FK506) was administered at 1 d prior to the procedure followed by methylprednisolone for 1 d and methotrexate for 4 d (on days 1, 3, 6, and 11) after BMT. Nonetheless, acute GVHD with oral mucositis developed on day 21; she was treated with additional FK506 and prednisolone. At 44 d after BMT, GVHD and oral mucositis had resolved somewhat. However, at day 64, a nodule transiently appeared in the lower lip mucosa; another nodule appeared in the buccal mucosa. The patient at 1 year and 5 mo of age was referred to our department for ongoing care.
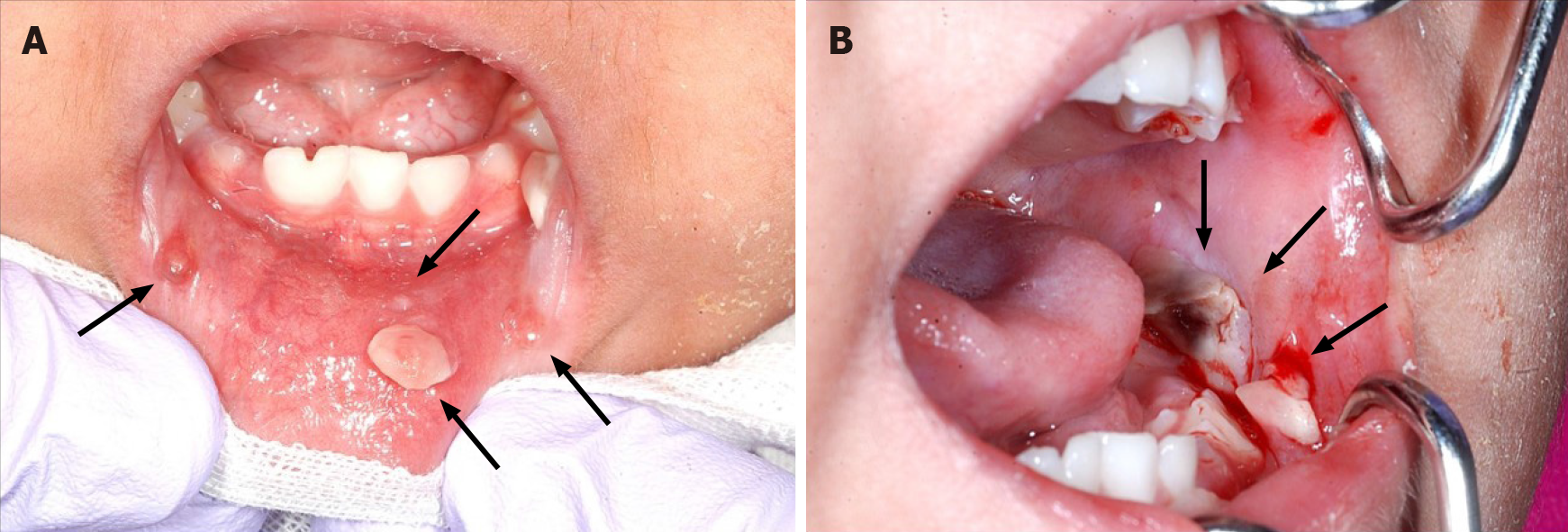
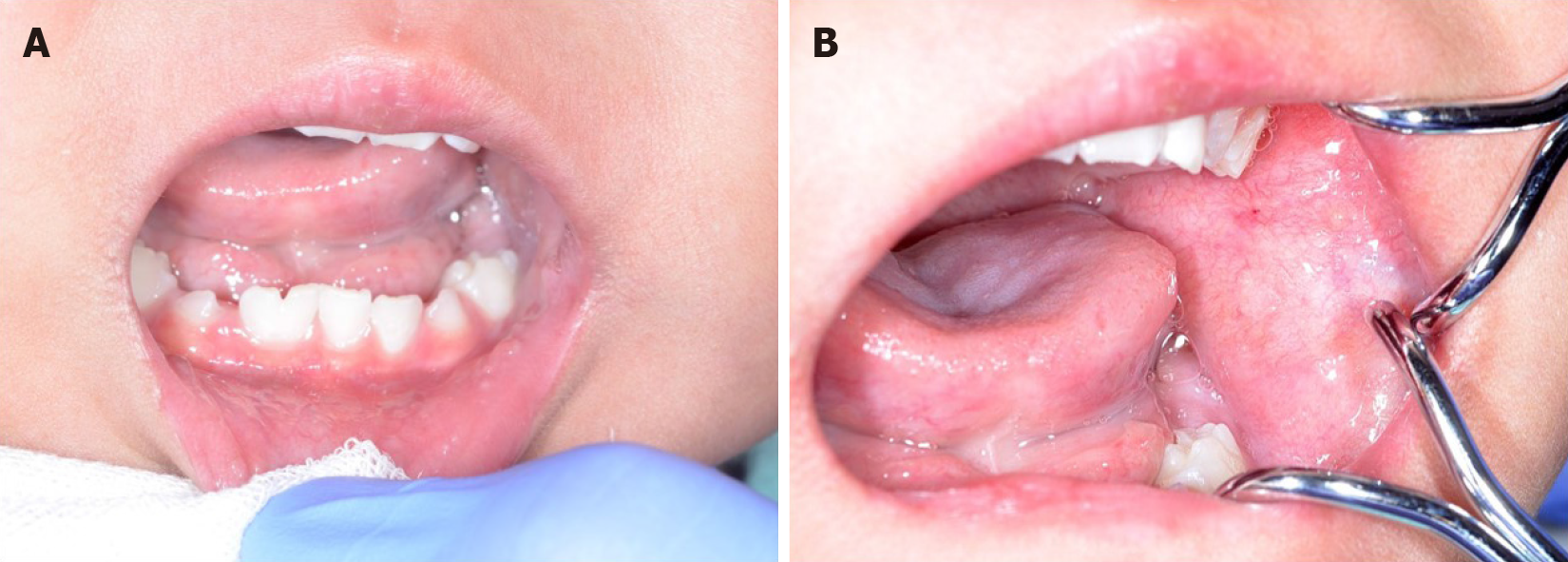
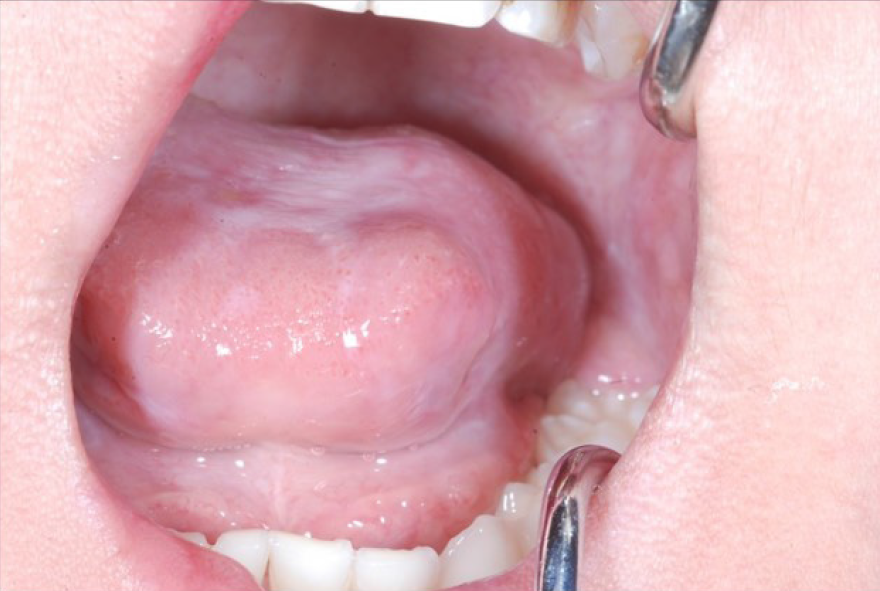
On presentation, the patient had bleeding oral mucositis in the lip and buccal regions. There were no findings suggestive of lichen planus. Pedunculated nodules 8 mm × 5 mm in size and 2 mm × 2 mm in size were identified in the lower lip mucosa. The left buccal mucosa was grayish-white in color, with several easily bleeding nodules (Figure 1). The platelet count on this first visit was below the normal limits at 5 × 104/μL. However, 1 mo after the first visit, all lesions spontaneously reduced (Figure 2). After 1 mo, GVHD recurred with oral mucositis. Her condition improved in response to elevated doses of both FK506 and prednisolone; however, GVHD-related gastrointestinal symptoms, mainly diarrhea and bloody stool, still persisted. Approximately 1 mo later, a nodule was detected on the left border of her tongue. Upon return to our department, an elastic soft, pedunculated nodule 12 mm × 10 mm in size was found on the left tongue border; this was diagnosed as a benign tumor (Figure 3). As her platelet count had sufficiently recovered (to 18.5 × 104/μL), the nodule was resected under general anesthesia. The procedure included 3-mm margins and excision into the muscle layer.
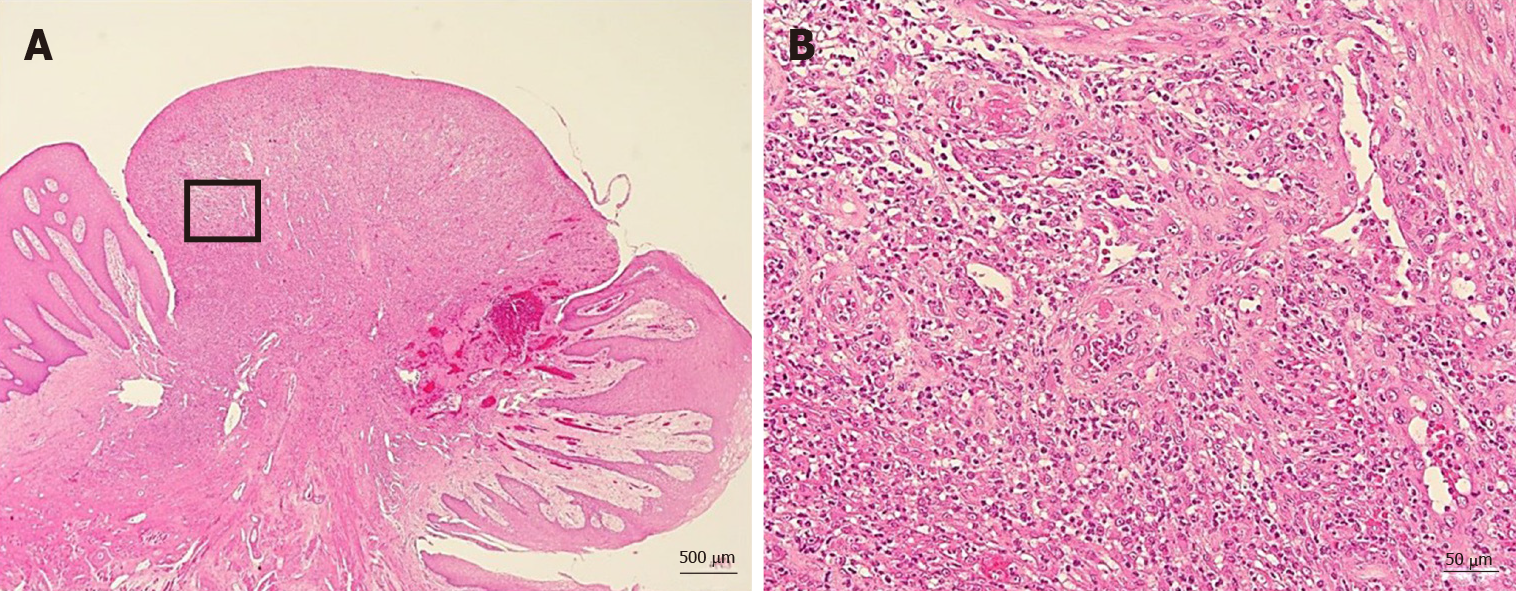
The tumor pathology was notable for granulation tissue consisting of capillaries and fibroblasts accompanied by moderate infiltration with inflammatory cells, including neutrophils. No malignancy was reported nor were there any inclusion bodies suggestive of cytomegalovirus (CMV) infection (Figure 4). A deoxyribonucleic acid test to detect CMV was performed on the excised tissue, but the results were negative.
The final diagnosis of the presented case was granuloma of the tongue caused by calcineurin inhibitor used for the treatment of oral GVHD.
After the excision, follow-up was performed without additional treatment.
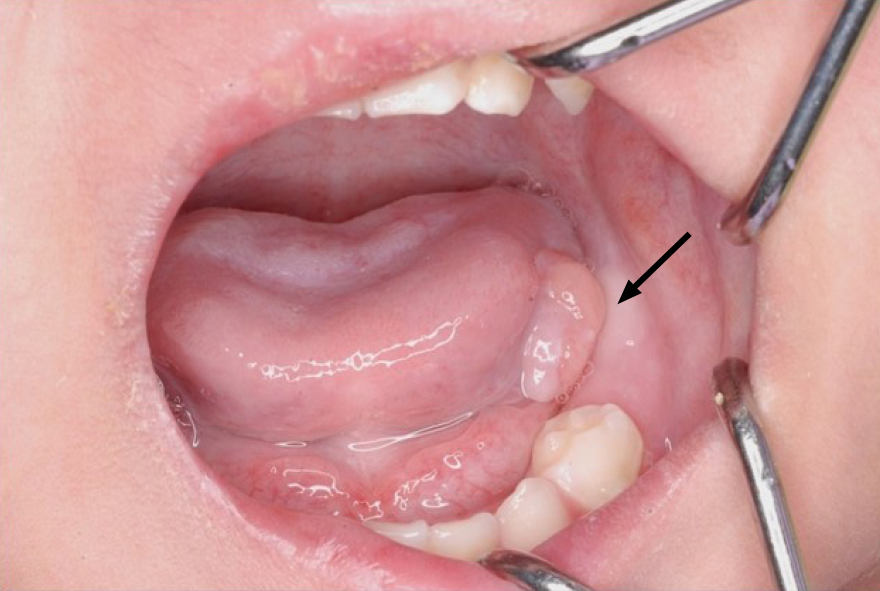
There was no recurrence of the lesion up to 1 year and 5 mo after the excision (Figure 5). However, the patient subsequently died due to an infection associated with PID.
GVHD associated with hematopoietic stem cell transplantation can be classified as acute or chronic. In this patient, GVHD developed 21 d after BMT and as such was diagnosed as acute GVHD. After several rounds of gastrointestinal symptoms, the diagnosis transitioned to chronic GVHD.
Typical oral lesions associated with GVHD include mucosal lichenoid changes, damage to the salivary glands, and trismus secondary to sclerosis[1]. In this patient, oral mucositis mainly comprised granulomatous nodules.
Woo et al[2] reported on non-gingival soft tissue granulomatous lesions in patients who developed GVHD after BMT. The features are as follows: (1) A non-gingival soft tissue nodule that exhibits rapid growth, raising malignancy concerns; (2) The presence of a certain degree of oral chronic GVHD in the tissues around the nodule; and (3) The presence of a certain degree of systemic GVHD, for which the patient was being treated with cyclosporin A (CsA)[2].
Among the explanations for these observations, when oral mucositis is caused by GVHD, the fibroblasts of the oral mucosa are activated, and lesions can result from the actions of CsA, which has a proliferative impact on fibroblasts[2,3].
In the present case, granulomatous nodules were detected in the non-gingival oral mucosa within a short time after the onset of GVHD. Initially, nodules were identified on the lower lip and left buccal mucosa; however, they spontaneously disappeared. Later, a nodule emerged at the left tongue border and was surgically removed. Prior to the appearance of these nodules, exacerbation of oral GVHD was observed.
In this case, FK506 was administered in an attempt to prevent and later treat GVHD instead of CsA. FK506 is a calcineurin inhibitor with a similar mechanism of action as CsA. CsA promotes fibroblast proliferation and collagen and glycosaminoglycan synthesis and likewise suppresses their degradation, resulting in connective tissue growth[4].
To our knowledge, 15 cases of transplant-related calcineurin inhibitor-induced oral inflammatory nodules with chronic and oral GVHD have been reported[5-7] (Table 1). The age at transplant was between 11 mo and 50 years, and the present case was the youngest. There were 12 cases where CsA was used as a calcineurin inhibitor and 3 cases where FK506 was used.
| Year | Ref. | Age at transplant | Sex | Primary disease | ChronicGVHD | Oral GVHD | Calcineurininhibitor | Location | Treatment | Malignancy | CMV |
| 1994 | Lee et al[3] | 19 | M | AML | O | O | CsA | Buccal mucosa | Excision | No | Negative |
| 1994 | Lee et al[3] | 45 | M | CLL | O | O | CsA | Buccal mucosa | Excision | No | Negative |
| 1994 | Woo et al[2] | 36 | F | AML | O | O | CsA | Buccal mucosa, lower lip | Excision | No | Negative |
| 1996 | Woo et al[2] | 27 | F | CML | O | O | CsA | Buccal mucosa | Excision | No | Not stated |
| 1996 | Woo et al[2] | 31 | M | AML | O | O | CsA | Tongue | Excision | No | Not stated |
| 1996 | Woo et al[2] | 50 | F | Myelodysplasia | O | O | CsA | Buccal mucosa | Excision | No | Not stated |
| 1996 | Woo et al[2] | 29 | F | AML | O | O | CsA | Buccal mucosa | Excision | No | Not stated |
| 1996 | Woo et al[2] | 33 | F | CML | O | O | CsA | Tongue | Excision | No | Not stated |
| 1996 | Woo et al[2] | 34 | M | AML | O | O | CsA | Tongue | Excision | No | Not stated |
| 2007 | Al-Mohaya et al[5] | 8 | M | ALL | O | O | CsA | Tongue | Excision | No | Not stated |
| 2007 | Al-Mohaya et al[5] | 3 | M | PNP deficiency | O | O | FK506 | Tongue | Excision | No | Not stated |
| 2009 | Suh et al[6] | 46 | M | Multiple myeloma | O | O | CsA | Tongue | Excision | No | Not stated |
| 2016 | Cheney-Peters et al[7] | 9 and 11 | M | Fanconi animia | O | O | FK506 | Tongue | Excision | No | Not stated |
| 2016 | Cheney-Peters et al[7] | 19 | M | Hodgkin's disease | O | O | CsA | Buccal mucosa | Excision | No | Not stated |
| 2020 | Present case | 11 mo and 1 | F | PID | O | O | FK506 | Buccal mucosa, lower lip, tongue | Natural disappearance or excision | No | Negative |
On the other hand, Terasawa et al[8] reported the case of a patient with GVHD with a nodule on the lower lip associated with CMV infection. In this case, we examined the nodule that tested negative for CMV. However, it is possible that CMV was involved in generating the earlier lesions that spontaneously disappeared. In Table 1, only four cases, including our case, checked for CMV. Therefore, it is necessary to consider CMV infection when examining the presence of granulomatous nodules with GVHD.
Based on our findings, our conclusion is that the tongue granuloma reported in this patient is most likely the result of fibroblast activation during recovery from oral GVHD exacerbated by the proliferative actions of the calcineurin inhibitor FK506.
Patients with GVHD have a high risk of developing secondary tumors typically within 5 to 10 years following BMT[9]. Furthermore, immunosuppressive agents and steroids administered prophylactically are also risk factors of secondary cancers. Therefore, it is necessary to follow-up with patients who develop GVHD to detect any malignancies as early as possible.
We experienced a rare case of granuloma on the tongue of a pediatric patient with GVHD. The differential diseases include malignant tumor and CMV infection. However, these diseases do not have unique clinical features and can only be differentiated via histopathological examination and quantitative deoxyribonucleic acid testing of the diseased tissue. In particular, if GVHD is present at the base and if immunosuppressive agents and steroids are being administered, it is extremely important to distinguish it from malignant tumors.
The authors would like to thank Dr. Mari Tanaka from the Department of Pediatrics, Tokyo Medical and Dental University, for her kind of this case.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gong N S-Editor: Zhang L L-Editor: A P-Editor: Liu JH
| 1. | Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ, Martin P, Chien J, Przepiorka D, Couriel D, Cowen EW, Dinndorf P, Farrell A, Hartzman R, Henslee-Downey J, Jacobsohn D, McDonald G, Mittleman B, Rizzo JD, Robinson M, Schubert M, Schultz K, Shulman H, Turner M, Vogelsang G, Flowers ME. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11:945-956. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2645] [Cited by in F6Publishing: 2726] [Article Influence: 151.4] [Reference Citation Analysis (0)] |
| 2. | Woo SB, Allen CM, Orden A, Porter D, Antin JH. Non-gingival soft tissue growths after allogeneic marrow transplantation. Bone Marrow Transplant. 1996;17:1127-1132. [PubMed] [Cited in This Article: ] |
| 3. | Lee L, Miller PA, Maxymiw WG, Messner HA, Rotstein LE. Intraoral pyogenic granuloma after allogeneic bone marrow transplant. Report of three cases. Oral Surg Oral Med Oral Pathol. 1994;78:607-610. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 34] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 4. | Arora PD, Silvestri L, Ganss B, Sodek J, McCulloch CA. Mechanism of cyclosporin-induced inhibition of intracellular collagen degradation. J Biol Chem. 2001;276:14100-14109. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 5. | Al-Mohaya M, Treister N, Al-Khadra O, Lehmann L, Padwa B, Woo SB. Calcineurin inhibitor-associated oral inflammatory polyps after transplantation. J Oral Pathol Med. 2007;36:570-574. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Suh JD, Blackwell KE, Nabili V. Graft-versus-host disease of the tongue. Otolaryngol Head Neck Surg. 2009;140:272-273. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 7. | Cheney-Peters D, Lund TC. Oral Pyogenic Granuloma after Bone Marrow Transplant in the Pediatric/Adolescent Population: Report of 5 Cases. J Pediatr Hematol Oncol. 2016;38:570-573. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Terasawa F, Shirozu T, Moon M, Tatematsu T, Kouketsu A, Ishibashi K, Maeda M, Kaetsu A. Intraoral pseudotumor of lower lip mucosa due to cytomegalovirus infection after bone marrow transplantation in a leukemia patient: A case report. J Oral Maxillofac Surg Med Pathol. 2013;25:167-170. [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 9. | Demarosi F, Soligo D, Lodi G, Moneghini L, Sardella A, Carrassi A. Squamous cell carcinoma of the oral cavity associated with graft vs host disease: report of a case and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;100:63-69. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 38] [Article Influence: 2.1] [Reference Citation Analysis (0)] |